A crystallization method for improving bulk density, fluidity and preparing non-agglomerated azithromycin
A technology of azithromycin and fluidity, which is applied in the field of increasing bulk density, fluidity and preparing non-agglomerated azithromycin crystals, which can solve the problems of small bulk density and poor fluidity, and achieve good fluidity, easy industrial production, and high purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
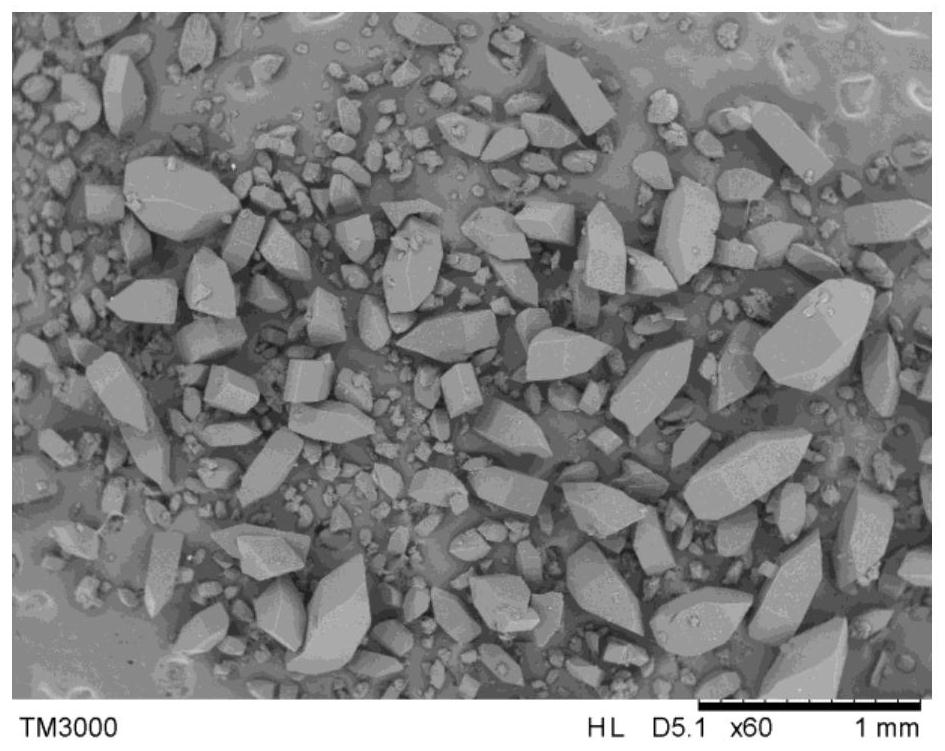
[0028] Add 8g of azithromycin to 24g of ethyl formate, heat to 60°C and keep it warm until the azithromycin is completely dissolved, quickly cool down to 50°C at a cooling rate of 30°C / h, add 0.04g of azithromycin seed crystals with a total weight of 0.5%, and grow crystals at constant temperature 0.5h, control the cooling rate at 12.5°C / h and slowly drop to 0°C after 4h, filter the crystals, and dry to obtain the azithromycin product. The microscope photo of the product is attached figure 1 , it can be seen from the figure that the azithromycin crystal grows well without agglomeration; the bulk density of the tested particles is 0.65g / mL; the angle of repose is 32.8°; the purity reaches 99.72%.
Embodiment 2
[0030] Add 20g of azithromycin to 20g of ethyl acetate, heat to 90°C and keep it warm until the azithromycin is completely dissolved, then quickly cool down to 70°C at a cooling rate of 60°C / h, add 0.01g of azithromycin seed crystals with a total weight of 0.05%, and grow crystals at constant temperature 20min, control the cooling rate to 8°C / h and drop to 30°C after 5h, filter and dry the crystals to obtain azithromycin product, the microscopic photo of the product is attached figure 2 , it can be seen from the figure that the azithromycin crystals grow well without agglomeration; the bulk density of the tested particles is 0.63g / mL; the angle of repose is 33.4°; the purity reaches 99.65%.
Embodiment 3
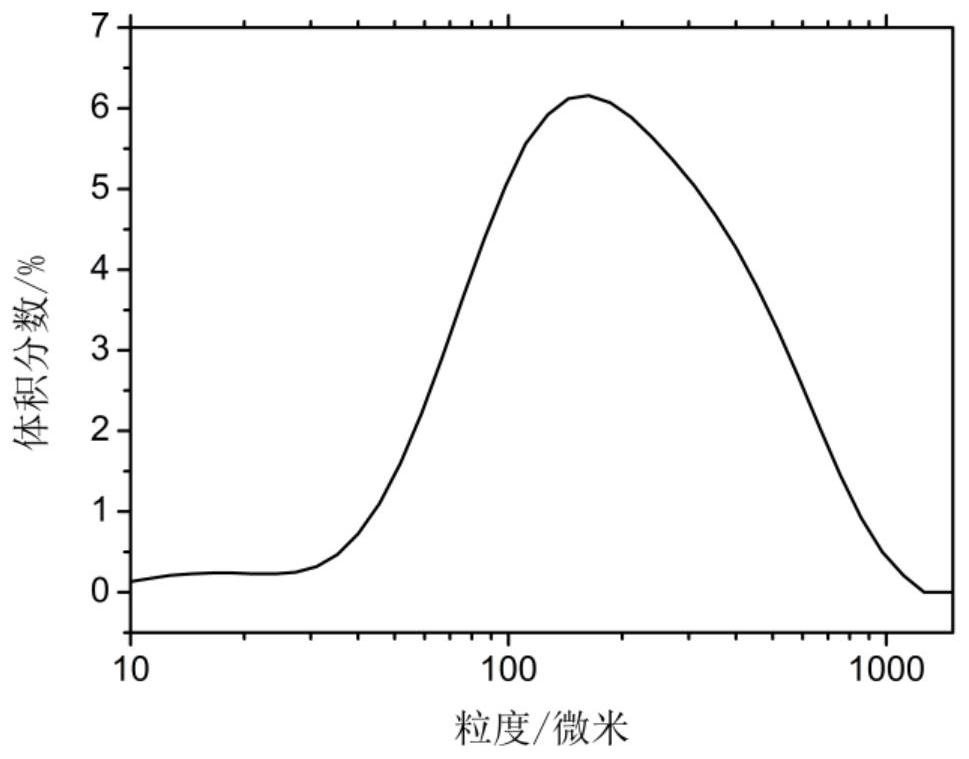
[0032] Add 10g of azithromycin to 50g of methyl acetate, heat it to 50°C and keep it warm until the azithromycin is completely dissolved, then quickly cool down to 40°C at a cooling rate of 30°C / h, add 0.5g of 5% seed crystals of the total weight of azithromycin, and culture at constant temperature crystallize for 40 minutes, control the cooling rate to 17.5°C / h and drop to 5°C after 2h, filter and dry the crystals to obtain the azithromycin product, and the microscopic photos of the product show that the azithromycin crystals grow well without agglomeration. The particle size distribution of the product is attached image 3 As shown, it can be seen from the figure that the particles are in a unimodal distribution; the main particle size is about 200 microns; the bulk density of the tested particles is 0.60g / mL; the angle of repose is 34.0°; the purity reaches 99.69%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com