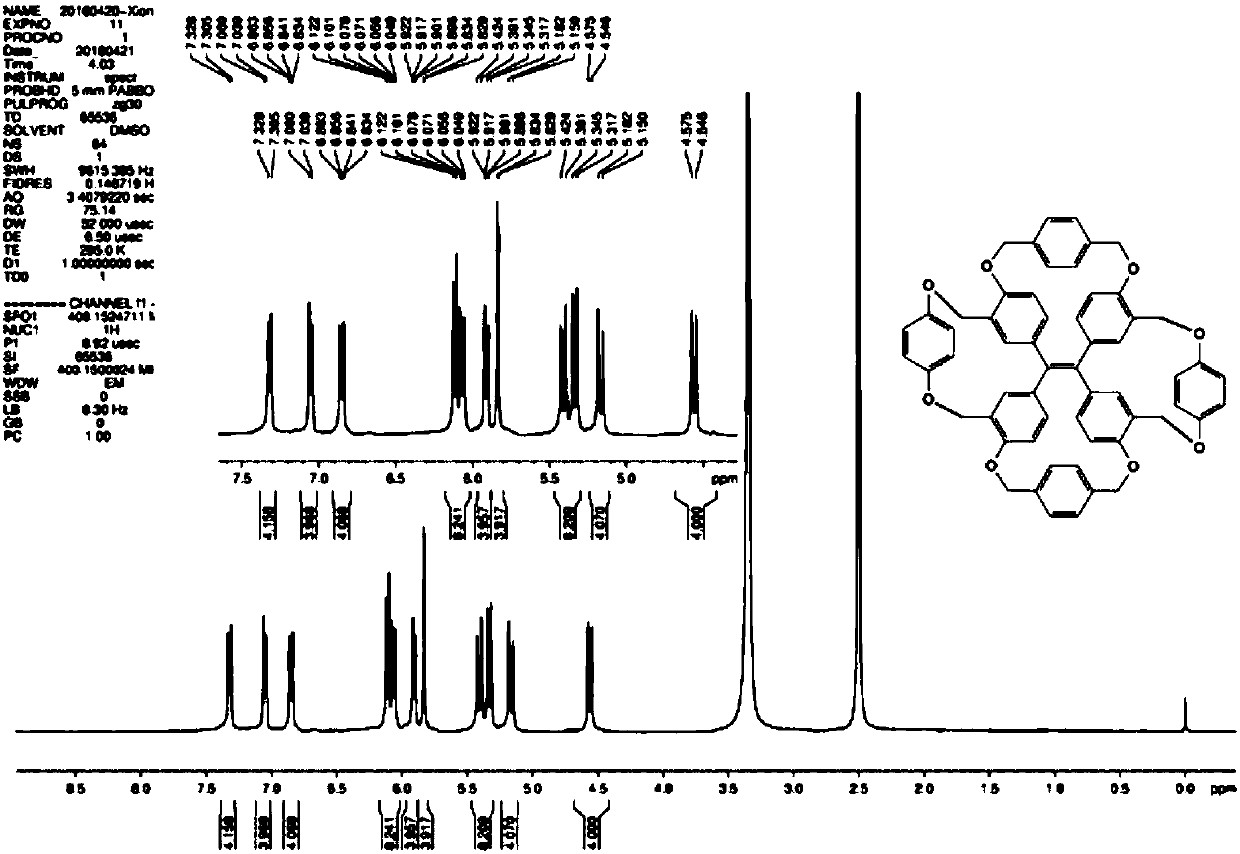
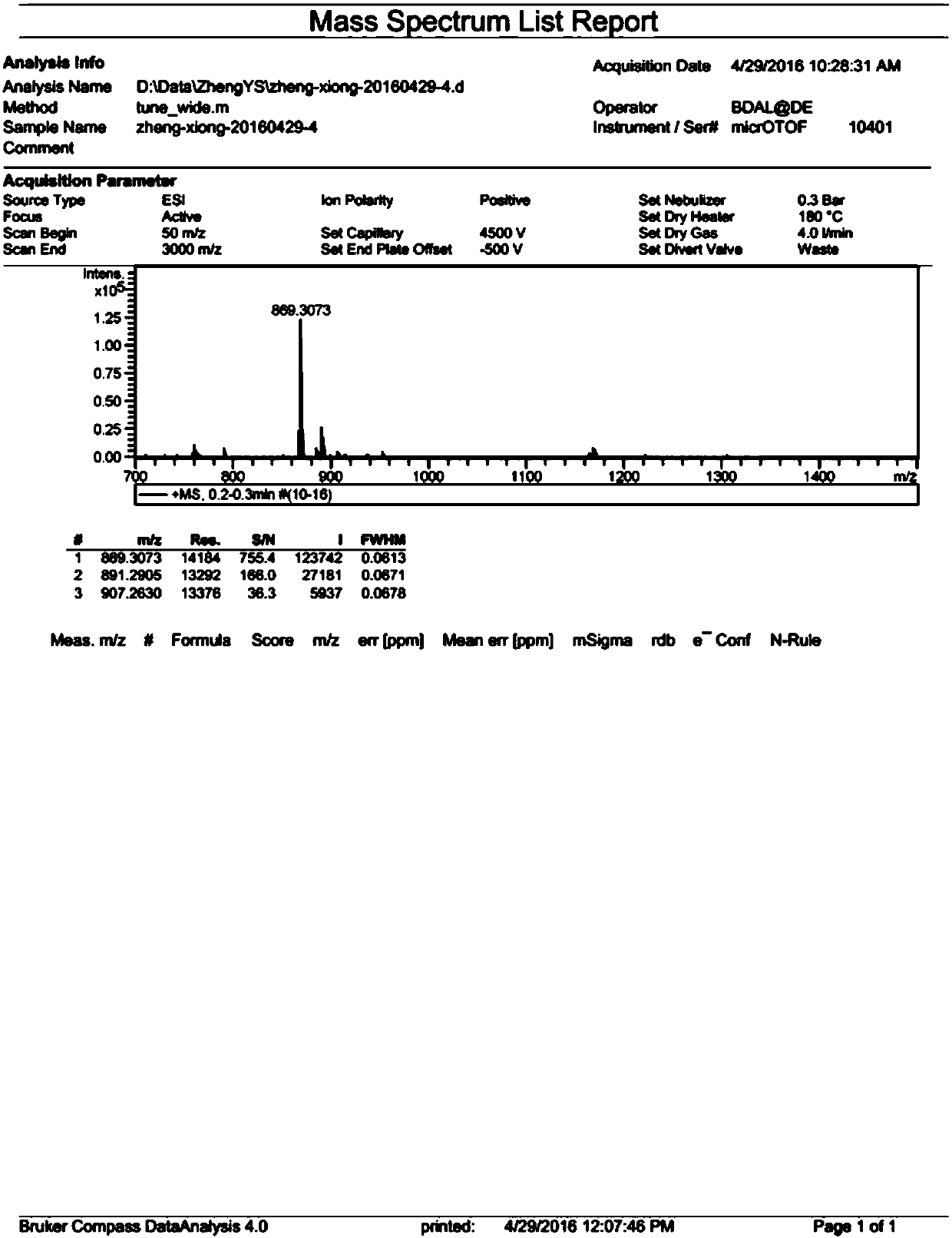
Method for fixing tetraphenyl ethylene (TPE) propeller conformation and splitting into single helicoids
A single, left-helical technology, applied in the chemical field, can solve the problems that the propeller structure is not easy to fix, and the helical chirality of AIE molecules has not been developed and applied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Tetraphenylethylene propeller type molecule I (in general formula I, X=O; R 1 = H; R 2 =H) synthesis:
[0063]
[0064] Add 0.5-2g of the compound of formula (II), 1-2g of hexamethylenetetramine and 10-50mL of trifluoroacetic acid into a round bottom flask, heat the reaction mixture under reflux for about 3 hours, and detect the reaction with TLC until the raw materials disappear. Add ice water, stir at room temperature for 2-4h, and extract with ethyl acetate. After the extracts were combined, they were dried with anhydrous sodium sulfate, filtered, and distilled under reduced pressure. The obtained crude product was separated and purified by silica gel column chromatography (eluent: ethyl acetate / petroleum ether 1:4 volume ratio), and the yellow formula (III) compound, yield 70%.
[0065] Add 200-800mg of the compound of formula (III), 300-900mg of 1,4-dibromomethylbenzene, 300-700mg of anhydrous potassium carbonate, and 50-300mL of dry acetonitrile into the rea...
Embodiment 2
[0071]Prepare a high-pressure liquid chromatography column with chirality, and the mobile phase is a dichloromethane-methanol mixed solvent with a volume ratio of 80:20. The compound of formula (I) can be resolved into two peaks, left-helical and right-helical. The retention of peak 1 The time is 2.455min, and the retention time of peak 2 is 3.430min. The percent enantiomeric excess of the two single helices of peak 1 and peak 2 is greater than 99%. Measured by single crystal x-ray diffraction, the compound of formula (I) comprises an equal amount of left-helical body and right-helical body, which is a racemate; the corresponding molecular structure of peak 1 is the left-helical body in the compound of formula (I), called It is M-I; peak 2 is right-helical, called P-I. For the single crystal structure of the compound of formula (I) and M-I, see Figure 4 with Figure 5 .
Embodiment 3
[0073] In tetrahydrofuran at a concentration of 5 mg / mL, the M-I specific rotation [α] 20 D =+479°; P-I specific rotation [α] 20 D =-502°. Absolute fluorescence quantum yield in tetrahydrofuran: formula (I) compound 79%; M-I is 94%, and P-I is also 94%; Be the absolute fluorescence quantum yield in 95:5 water / THF suspension in volume ratio: formula (I) compound 88%; M-I is 85%, P-I is also 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com