Nucleic acid sequence for coding human choline acetyl transferase or fusion protein of human choline acetyl transferase and application of nucleic acid sequence
A technology of nucleic acid sequence and coding sequence, which is applied in the field of nucleic acid sequence encoding human choline acetyltransferase or its fusion protein, and preparation of transduction peptide-human choline acetyltransferase fusion protein, which can solve the problem of easy formation of inclusion bodies The expression level and the difficulty in expressing human choline acetyltransferase have achieved the effects of safe clinical medication, high enzyme activity and high protein purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Construction of the recombinant expression vector of the transduction peptide-ChAT fusion protein
[0079] According to ChAT nucleic acid sequence (SEQ ID NO:1) and transduction peptide nucleic acid sequence (SEQ ID NO:3) and Linker nucleic acid sequence (SEQ ID NO:5), the whole gene (SEQ ID NO:7) was synthesized, and its two ends It contains Nco I and BamH I restriction sites, and is inserted into the fusion protein expression vector pET15b vector (Novagen Company). The resulting recombinant vector was named pET15b-PTD-ChAT. The gene sequencing analysis was correct. (Shanghai Shengong Biological Engineering Company).
Embodiment 2
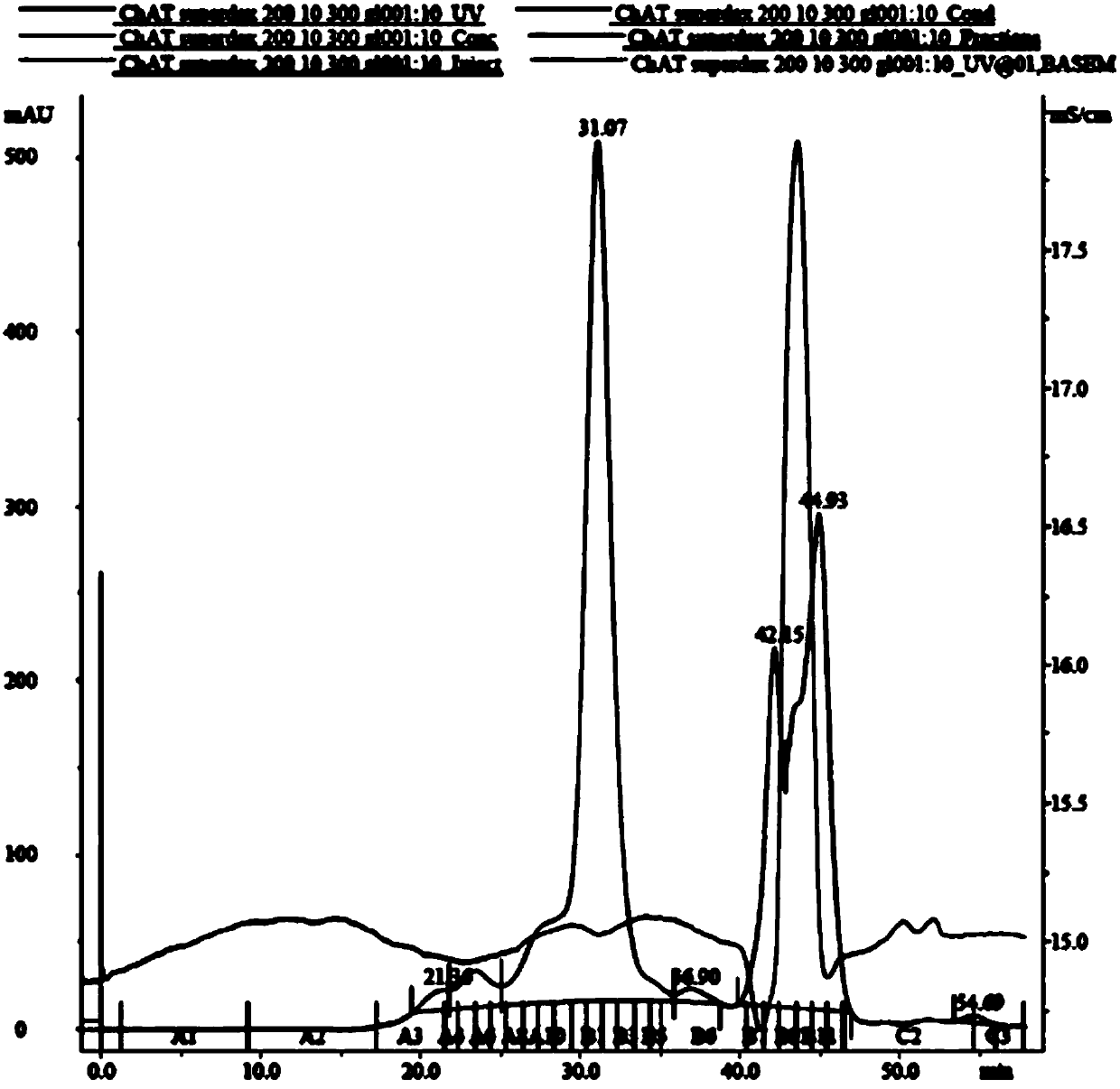
[0080] Example 2: Expression and purification of transduction peptide-ChAT fusion protein
[0081] The recombinant expression vector pET15b-PTD-ChAT obtained in Example 1 was transformed into Escherichia coli strain Ecoli.BL21(DE3), and the recombinant strain was obtained by screening, which was named E.coli BL21(DE3) / pET15b-PTD-ChAT. for protein expression and purification. Can be stored frozen.
[0082] After resuscitating and activating the recombinant strains obtained above or freezing the recombinant strains, spread them evenly on the LB solid medium plate, and put them into a constant temperature incubator at 37°C for overnight cultivation. A single colony was picked from the plate and inoculated into LB liquid medium (containing Amp+, 100 μg / ml), and cultured overnight at 37°C and 220 r / min as the primary seed. The first-grade seeds were inoculated into 5 kinds of medium (including Amp+) with a volume fraction of 5%, and continued to culture to a certain cell concen...
Embodiment 3
[0132] Example 3: Optimization of Transduction Peptide-ChAT Formulations
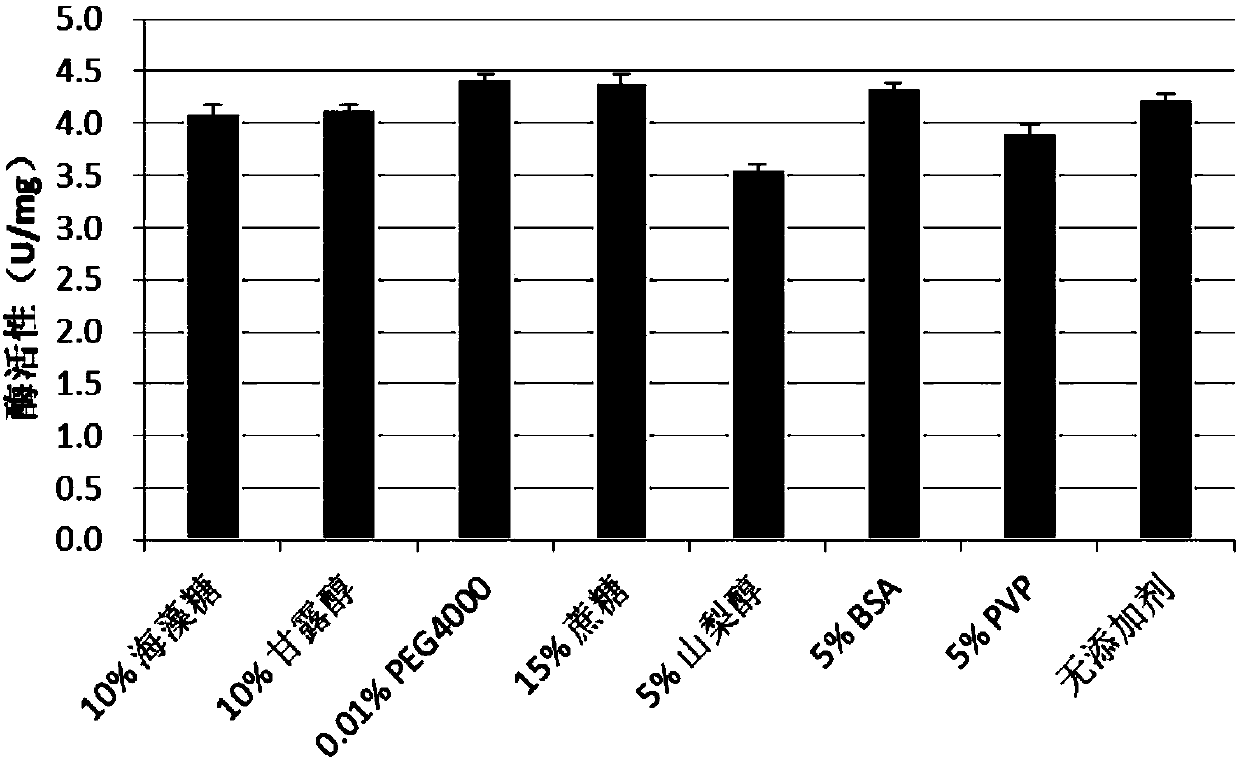
[0133] The prepared transduction peptide-ChAT stock solution was made into freeze-dried powder after adding different concentrations of trehalose, mannitol, PEG4000, Tween 80, sorbitol or other protective agents (sucrose, PVP, BSA, etc.). Place it in a 37-degree incubator for 1 week, detect the activity of the transduction peptide-ChAT with reference to the method in Example 2 above, calculate the relative activity, further design the composition of the formula, and determine the formulation of the transduction peptide-ChAT, Determine the best storage method. Three samples were taken in each group, and the average value was calculated. The experimental setup and results are shown in Table 3 below and attached image 3 .
[0134] Table 3: Effects of different concentrations of protective agents on transduction peptide-ChAT
[0135]
[0136] Wherein, relative activity=(enzyme activity after test / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com