Method using wet method to treat high-fluorine and high-chlorine copper smelting dust containing zinc
A wet processing, high fluorine technology, applied in the field of heavy metal metallurgy, can solve the problems of limited removal of fluorine and chlorine, difficult recovery, increased labor costs and production costs, etc., achieving significant economic and social benefits, eliminating high COD, The effect of cost-effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
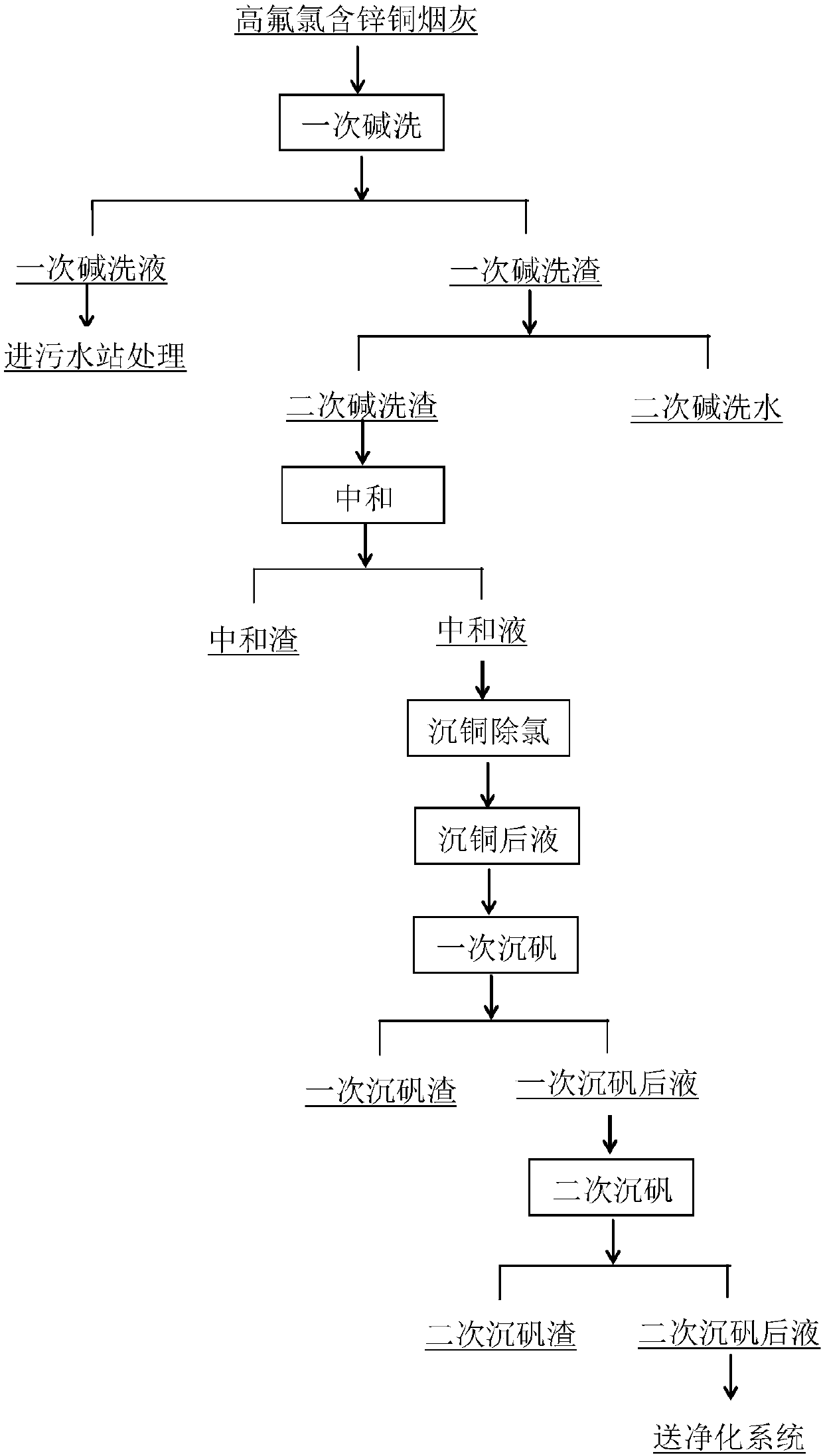
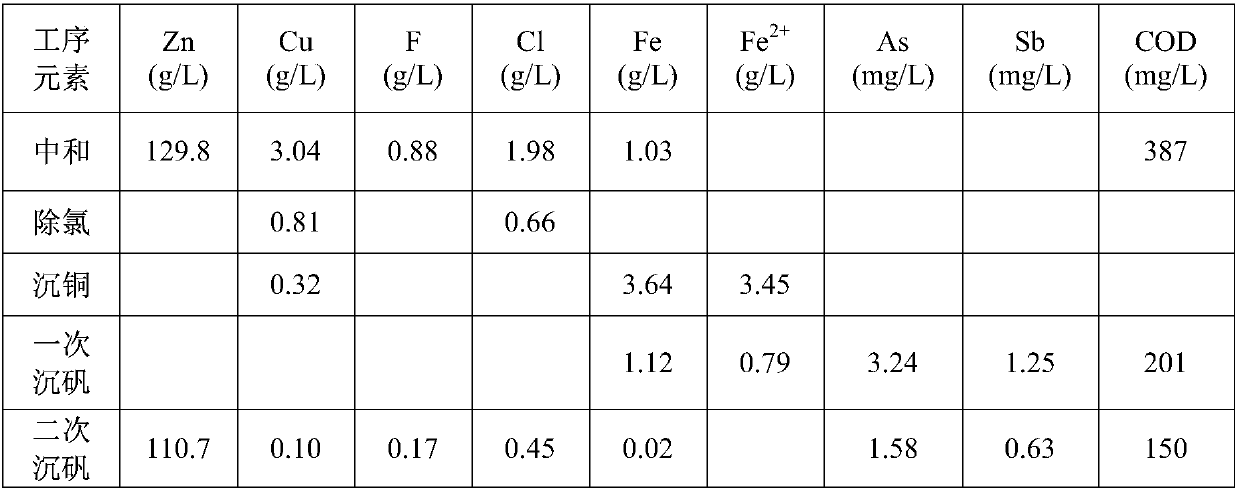
[0032] Take copper soot (Cu: 2.65wt%, Zn: 23.62wt%, F: 2.26wt%, Cl: 8.55wt%) and pour 8t into 50m 3 In the reaction tank, add 40m of river water3 , heat the reaction tank with steam to 75°C and stir the reaction, take 200L hydrogen peroxide and slowly add it to the solution, the addition time is controlled to 1.5h, add caustic soda during the reaction process, control the pH of the reaction end point to 9, and the reaction time is 2h, After the reaction, liquid-solid separation is carried out, the liquid enters the sewage station for treatment, and the slag is poured into another reaction tank for alkali secondary washing.
[0033] Pass 40m into the reaction tank equipped with alkali-washing slag 3 River water, heat the reaction tank with steam to 75°C and stir the reaction, add the additive sodium carbonate during the reaction, control the pH of the reaction end point to 9, and the reaction time is 2h. A part is taken for laboratory analysis, and the slag after alkali second...
Embodiment 2
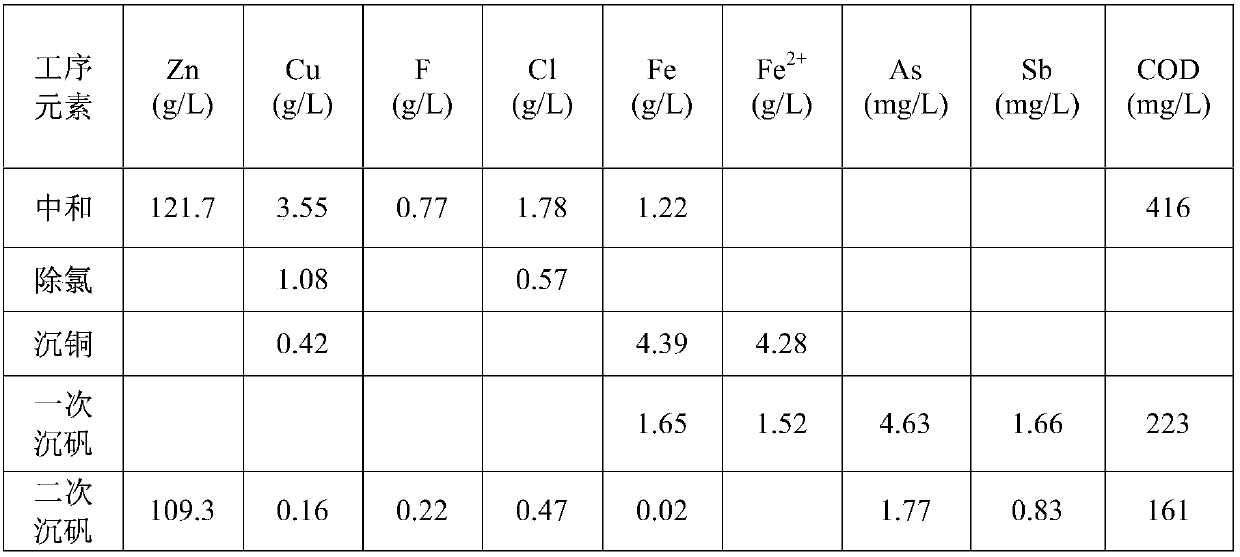
[0045] Take copper soot (Cu: 2.65%, Zn: 23.62%, F: 2.26%, Cl: 8.55%) and pour 8t into 50m 3 In the reaction tank, add alkali two washing liquid 40m in example 1 3 , heat the reaction tank with steam to 75°C and stir the reaction, take 200L hydrogen peroxide and slowly add it to the solution, the addition time is controlled to 1.5h, add caustic soda during the reaction process, control the pH of the reaction end point to 10, and the reaction time is 2h, After the reaction, liquid-solid separation is carried out, the liquid enters the sewage station for treatment, and the slag is poured into another reaction tank for alkali secondary washing.
[0046] Pass 40m into the reaction tank equipped with alkali-washing slag 3 River water, heat the reaction tank with steam to 75°C and stir the reaction, add the additive sodium carbonate during the reaction, control the pH of the reaction end point to 10, and the reaction time is 2h. A part is taken for laboratory analysis, and the slag...
Embodiment 3
[0058] Take copper soot (Cu: 3.21%, Zn: 29.96%, F: 1.96%, Cl: 5.23%) 8t and pour it into 50m 3 In the reaction tank, add 40m of river water 3 , heat the reaction tank with steam to 80°C and stir the reaction, take 250L hydrogen peroxide and slowly add it to the solution, the adding time is controlled to 1.5h, add caustic soda during the reaction, control the pH of the reaction end point to 8, and the reaction time is 2h, After the reaction, liquid-solid separation is carried out, the liquid enters the sewage station for treatment, and the slag is poured into another reaction tank for alkali secondary washing.
[0059] Pass 40m into the reaction tank equipped with alkali-washing slag 3 River water, heat the reaction tank with steam to 80°C and stir the reaction, add additive sodium carbonate during the reaction, control the pH of the reaction end point to 8, and the reaction time is 2h. A part is taken for laboratory analysis, and the slag after alkali secondary washing is ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com