Copper (I) phosphorine heterocyclic complex and preparation method and application thereof
A technology of benzene heterocycles and complexes, which is applied in the field of copper (I) phosphine benzene heterocycle complexes and its preparation, can solve the problems that the fluorescence intensity and lifetime cannot change with temperature, the temperature range is narrow, and the sensitivity is low, achieving excellent Effects of luminescence properties, high sensitivity, and excellent luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
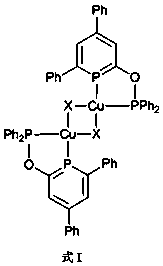
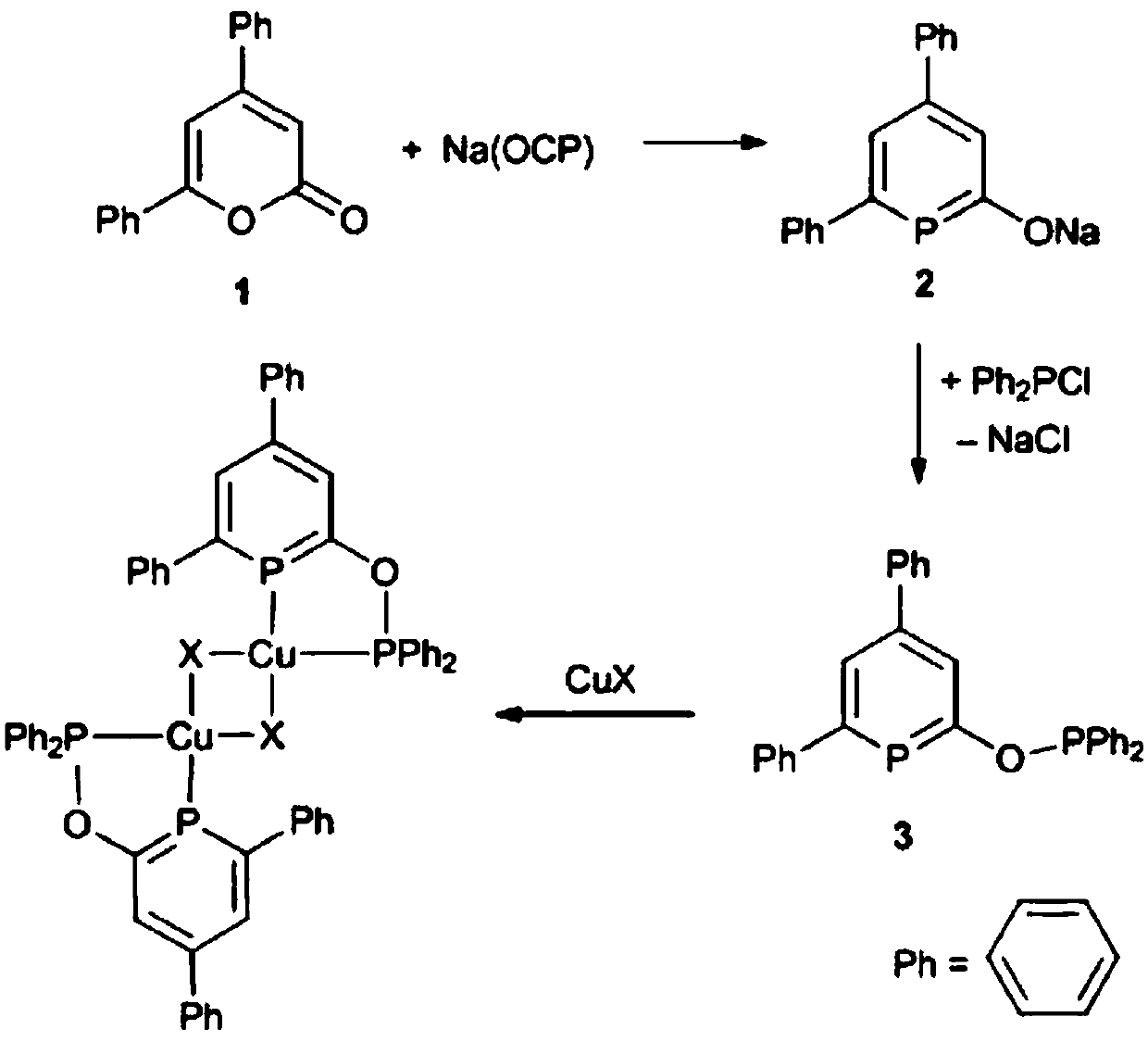
[0050] Example 1 Preparation of copper (I) chlorophosphine benzene heterocyclic bisphosphine complex
[0051] 1. Prepare the ligand
[0052] (1) Under nitrogen atmosphere, add (2.0g, 8.0mmol) 4,6-diphenyl-2-pyrone, (2.43 g, 8.0 mmol) [Na(OCP)·(dioxane) 2.5 ], and 50mL of anhydrous tetrahydrofuran were added to the reaction flask and heated to reflux at 90°C for 24 hours. After the reaction, cool, filter, extract with anhydrous tetrahydrofuran and anhydrous ether successively, and wash the solid with anhydrous n-hexane to obtain the corresponding intermediate product as light yellow solid powder (phen) 2 C 5 PONa. Yield: 73%. The melting point is 185°C. 1 H NMR (CD 3 CN, 400MHz): δ = 7.65 (d, 4 H, C arom ), 7.37 (t, 2 H, C arom ), 7.29 (m, 5 H, C arom &C 5 ),7.00 (d, 1 H, C 5 ); 13 C{ 1 H}NMR (CD 3 CN, 100.5 MHz): δ = 209.5 (d, C 5 ), 171.5 (d,C 5 ), 145.8(d, C 5 ) 145.2(s, C 5 ), 144.2(s, C 5 ), 128.8(d, C arom ), 127.6(s, C arom ),127.3 (d, C arom ), 127....
Embodiment 2
[0059] Example 2 Preparation of copper (I) bromophosphine benzene heterocyclic bisphosphine complex
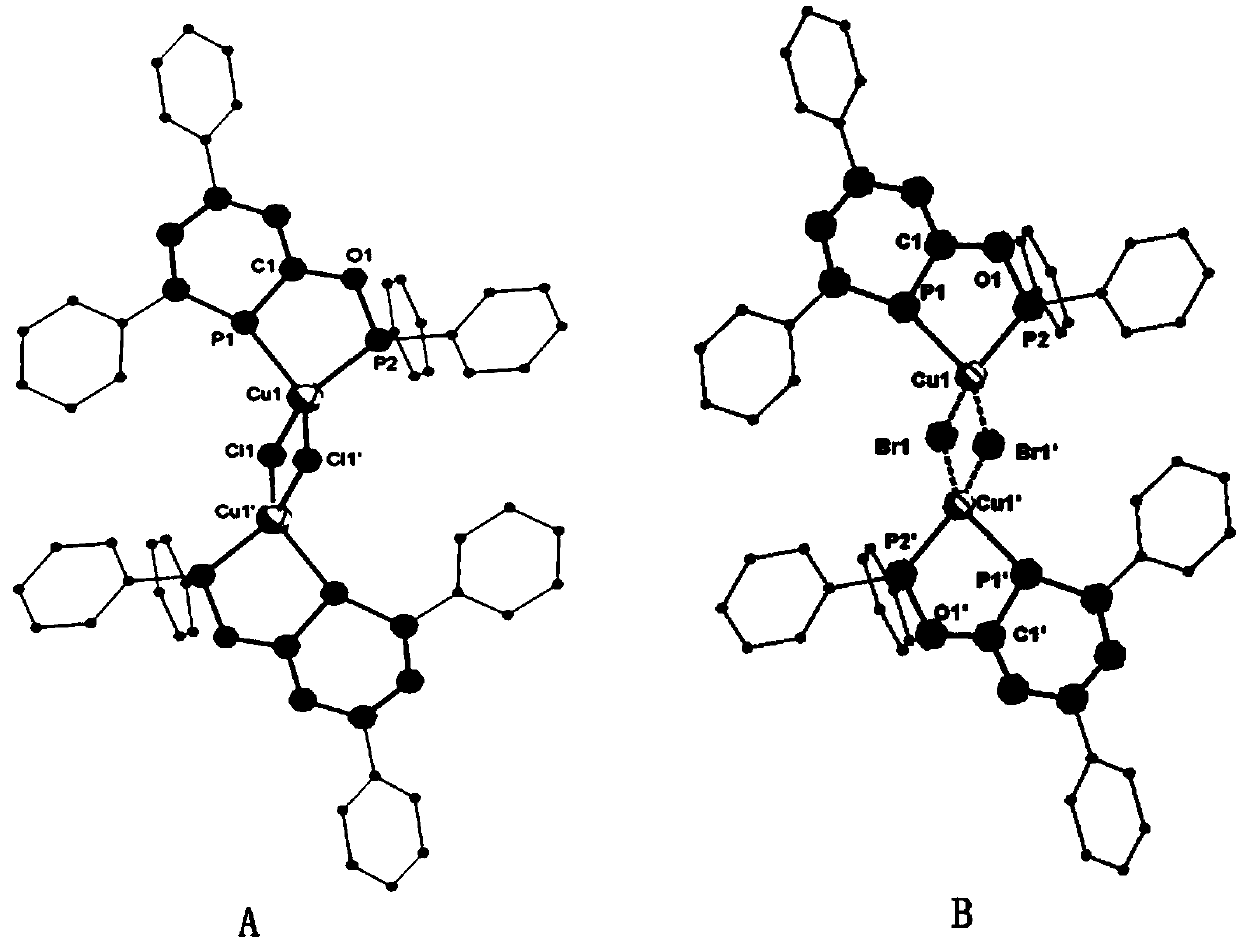
[0060] Ligand (phen) 2 C 5 P-O-P (phen) 2 The preparation is the same as in Example 1, under nitrogen atmosphere, the ligand (18.6 mg, 0.13 mmol) (phen) 2 C 5 P-O-P (phen) 2 React with (58.0 mg, 0.13 mmol) cuprous bromide CuBr in anhydrous tetrahydrofuran for 2 hours, filter to obtain a solid, and wash the solid with anhydrous n-hexane to obtain complex 2: [(phen) 2 C 5 P-O-P (phen) 2 ] 2 [Cu 2 Br 2 ]. Yield: 87%.
[0061] Anal. Calcd for C 58 h 44 P 4 o 2 Cu 2 Br 2 : C, 58.85%; H, 3.75%; Found: C, 58.82%; H, 3.88%.
[0062] UV / Vis: λ 1 = 328 nm, λ 2 = 410nm.
[0063] IR (ATR, [cm -1 ]): 3057 (w, C-H str.), 1965 (w), 1900 (w), 1814 (w), 1777(w), 1570 (m), 1531, 1492, 1472, 1450 (m, -C 6 h 5 ), 1436 (m), 1383 (m), 1353 (w), 1334 (w), 1312 (w), 1272 (w), 1249 (w), 1184 (w), 1138 (s, C-O str.) ,1107(s), 1079(m), 1027(w), 999(w), 957(s), 915(w), 898(w), ...
Embodiment 3
[0064] Example 3 Preparation of copper (I) iodophosphine benzene heterocyclic bisphosphine complex
[0065] Ligand (phen) 2 C 5 P-O-P (phen) 2 The preparation is the same as in Example 1, under nitrogen atmosphere, the ligand (13.6 mg, 0.07 mmol) (phen) 2 C 5 P-O-P (phen) 2 React with (32.0 mg, 0.07 mmol) cuprous iodide CuI in anhydrous tetrahydrofuran for 2 hours, filter to obtain a solid, and wash the solid with anhydrous n-hexane to obtain complex 3: [(phen) 2 C 5 P-O-P (phen) 2 ] 2 [Cu 2 I 2 ]. Yield: 90%.
[0066] Anal. Calcd for C 58 h 44 P 4 o 2 Cu 2 I 2 : C, 54.52%; H, 3.47%; Found: C, 54.21%, H, 3.45%.
[0067] UV / Vis: λ 1 = 332 nm, λ 2 = 410nm.
[0068] IR (ATR, [cm -1 ]): 3054 (w, C-H str.), 1956 (w), 1900 (w), 1817 (w), 1776(w), 1570 (m), 1532, 1492, 1471, 1450 (m, -C 6 h 5 ), 1436 (m), 1381 (m), 1353 (w), 1334 (w), 1313 (w), 1271 (w), 1248 (w), 1185 (w), 1135 (s, C-O str.) ,1107(s), 1079(m), 1068(w), 1028(w), 999(w), 957(s), 917(w), 899(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com