Aminophylline tablet and preparation method thereof
A technology of aminophylline and cholophylline, which is applied in the field of aminophylline tablets and its preparation, can solve problems such as narrow range of blood drug concentration, improper control of drug dosage, cramps and allergies, etc., and achieve mass production and enrich health care effects , Improve the effect of bad taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
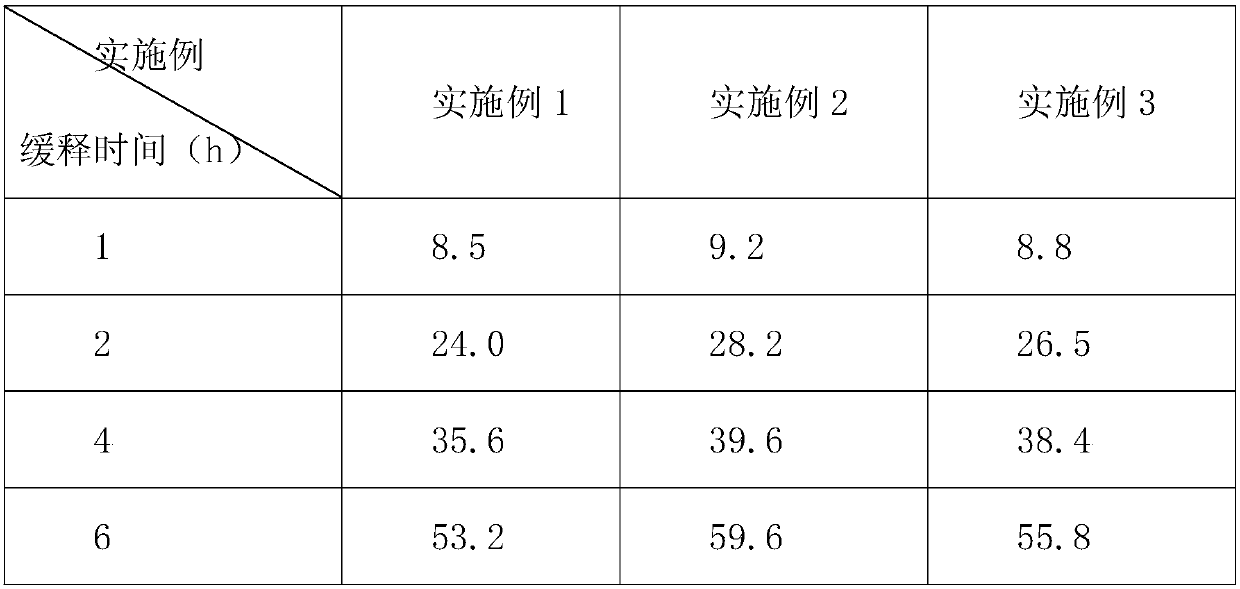
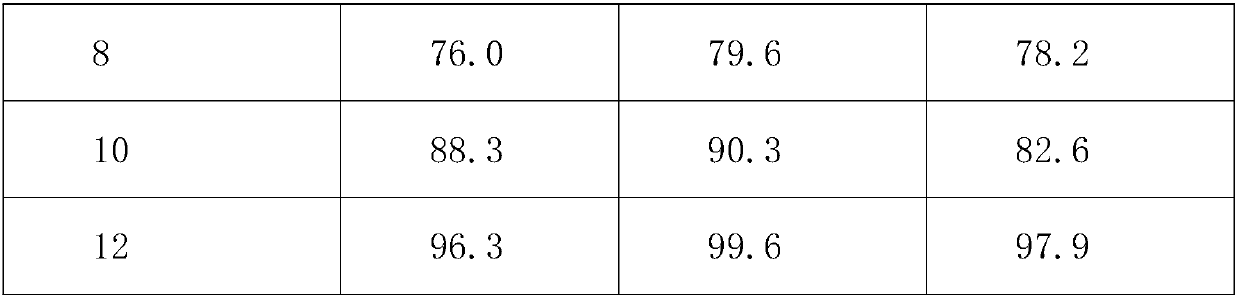
Embodiment 1
[0021] An aminophylline tablet, made of the following raw materials in weight ratio: 45 parts of aminophylline, 45 parts of cholophylline, 305 parts of povidone K, 5 parts of whole milk powder, 5 parts of silicon dioxide, and 5 parts of vitamin C , 5 parts of vitamin E, 3 parts of honey, 0.5 parts of mugwort leaves, 1 part of kudzu root, 2 parts of medicinal soybean oil, and 2 parts of carboxymethyl cellulose.
[0022] Among them, Artemisia argyi and Pueraria lobata root were first soaked in ethanol for 12 hours, rinsed with sterile water, dried and crushed to nanometer scale.
[0023] A preparation method of aminophylline tablet, comprising the steps of:
[0024] (1) Take the aminophylline and cholophylline of formula quantity, granulate with 80 mesh sieve, dry at 50-60 ℃;
[0025] (2) Weigh the formula amount of povidone K30, whole milk powder, silicon dioxide, vitamin c, vitamin e, honey, mugwort leaf, kudzu root, dispersant, slow release agent, add purified water, ultraso...
Embodiment 2
[0029] An aminophylline tablet, made of the following raw materials in weight ratio: 60 parts of aminophylline, 55 parts of cholophylline, 306 parts of povidone K, 6 parts of whole milk powder, 6 parts of silicon dioxide, and 6 parts of vitamin C , 6 parts of vitamin E, 4 parts of honey, 0.7 parts of mugwort leaves, 2 parts of kudzu root, 3 parts of medicinal soybean oil, and 3 parts of carboxymethyl cellulose.
[0030] Among them, Artemisia argyi and Pueraria lobata root were first soaked in ethanol for 12 hours, rinsed with sterile water, dried and crushed to nanometer scale.
[0031] A preparation method of aminophylline tablet, comprising the steps of:
[0032] (1) Take the aminophylline and cholophylline of formula quantity, granulate with 80 mesh sieve, dry at 50-60 ℃;
[0033] (2) Weigh the formula amount of povidone K30, whole milk powder, silicon dioxide, vitamin c, vitamin e, honey, mugwort leaf, kudzu root, dispersant, slow release agent, add purified water, ultras...
Embodiment 3
[0037] An aminophylline tablet, made of the following raw materials in weight ratio: 55 parts of aminophylline, 48 parts of cholophylline, 306 parts of povidone K, 6 parts of whole milk powder, 6 parts of silicon dioxide, and 6 parts of vitamin C , 6 parts of vitamin E, 4 parts of honey, 0.7 parts of mugwort leaves, 2 parts of kudzu root, 3 parts of medicinal soybean oil, and 3 parts of carboxymethyl cellulose.
[0038] Among them, Artemisia argyi and Pueraria lobata root were first soaked in ethanol for 12 hours, rinsed with sterile water, dried and crushed to nanometer scale.
[0039] A preparation method of aminophylline tablet, comprising the steps of:
[0040] (1) Take the aminophylline and cholophylline of formula quantity, granulate with 80 mesh sieve, dry at 50-60 ℃;
[0041] (2) Weigh the formula amount of povidone K30, whole milk powder, silicon dioxide, vitamin c, vitamin e, honey, mugwort leaf, kudzu root, dispersant, slow release agent, add purified water, ultras...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com