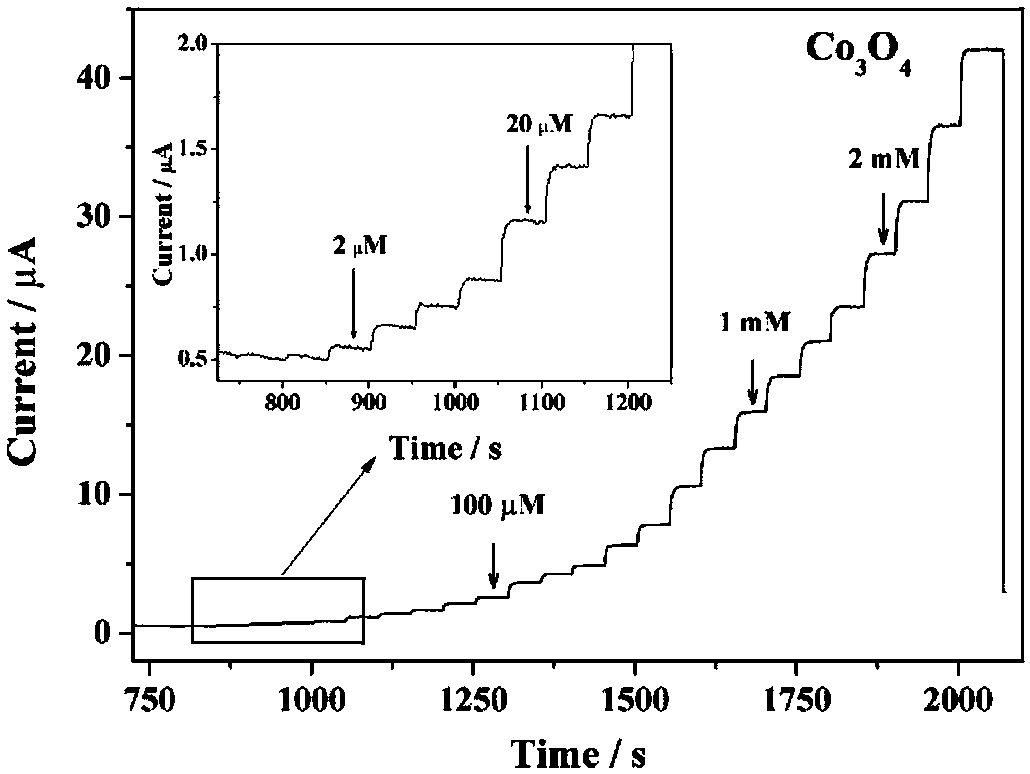
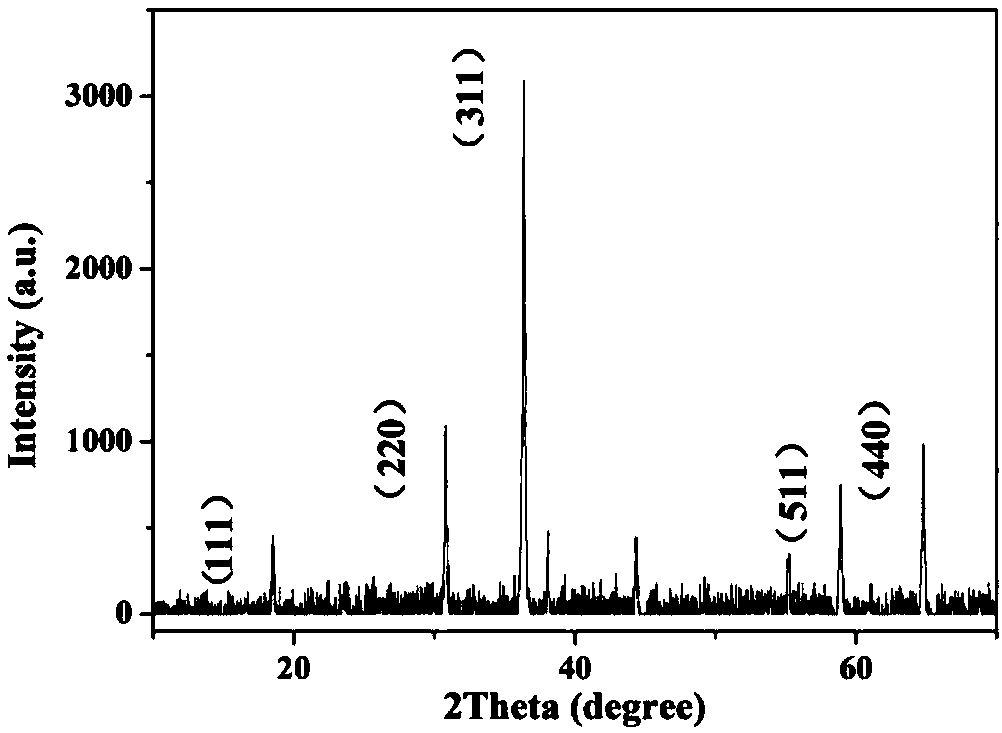
Preparation method of regularly octahedral cobalt oxide micro-nano-particle material
A technology of micro-nano particles and regular octahedrons, applied in the direction of cobalt oxide/cobalt hydroxide, etc., to achieve the effects of low energy consumption, simple process flow, and high electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention octahedral Co 3 o 4 The preparation method of micro-nano particle material, comprises the following steps:
[0031] S1. Weigh CoCl 2 ·6H 2 O was added to deionized water, where CoCl 2 ·6H 2 O and H 2 The mass ratio of O is 1:80.00~106.34, ultrasonically dispersed for 20~30min to form a uniform wine red solution;
[0032] S2. Weigh CO(NH 2 ) 2 Join in the solution prepared in step S1, wherein, CO(NH 2 ) 2 with CoCl 2 ·6H 2 The mass ratio of O is 4:5, ultrasonically dispersed for 20-30 minutes to obtain a uniform solution;
[0033] S3. Transfer the solution obtained in step S2 to a stainless steel autoclave lined with polytetrafluoroethylene, with a filling ratio of 50-60%, seal it, react at 150° C. for 4-12 hours, collect the precipitate, wash it, and vacuum-dry it. Get a pink precursor;
[0034] Preferably, the inner volume of the stainless steel autoclave is 32.8-36.4mL.
[0035] S4. Transfer the precursor obtained in step S3 to a m...
Embodiment 1
[0038] Weigh 0.2050g of CoCl 2 ·6H 2 O, added to 16.4 mL of deionized water, CoCl 2 ·6H 2 O and H 2 The mass ratio of O was 1:80.00, and ultrasonically dispersed for 20 minutes to form a uniform wine-red solution.
[0039] Subsequently, weigh 0.1640g of CO(NH 2 ) 2 Add to the above solution, ultrasonically disperse for 20min.
[0040] The above solution was transferred to a polytetrafluoroethylene-lined stainless steel autoclave with a volume of 32.8mL, the filling ratio was 50%, sealed, and reacted at 140°C for 4h, the precipitate was collected, washed and dried, and a pink precursor was obtained.
[0041] The above precursor was transferred to a muffle furnace, and the temperature was raised to 400 °C at a rate of 5 °C / min, and kept for 4 h.
[0042] Subsequently, naturally cooled to room temperature, the octahedral Co 3 o 4 micro-nanomaterials, such as figure 1 shown.
Embodiment 2
[0044] Weigh 0.2050g of CoCl 2 ·6H 2 O was added to 16.4 mL of deionized water, CoCl 2 ·6H 2 O and H 2 The mass ratio of O was 1:80.00, and ultrasonically dispersed for 25 minutes to form a uniform wine-red solution.
[0045] Subsequently, weigh 0.1640g of CO(NH 2 ) 2 Add to the above solution, ultrasonically disperse for 25min.
[0046] The above solution was transferred to a polytetrafluoroethylene-lined stainless steel autoclave with a volume of 32.8mL, sealed, and reacted at 150°C for 6h, and the precipitate was collected, washed and dried to obtain a pink precursor.
[0047] The above-mentioned pink precursor was transferred to a muffle furnace, and the temperature was raised to 400 °C at a rate of 5 °C / min, and kept for 4 h.
[0048] Subsequently, naturally cooled to room temperature, the octahedral Co 3 o 4 micro-nanomaterials, such as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com