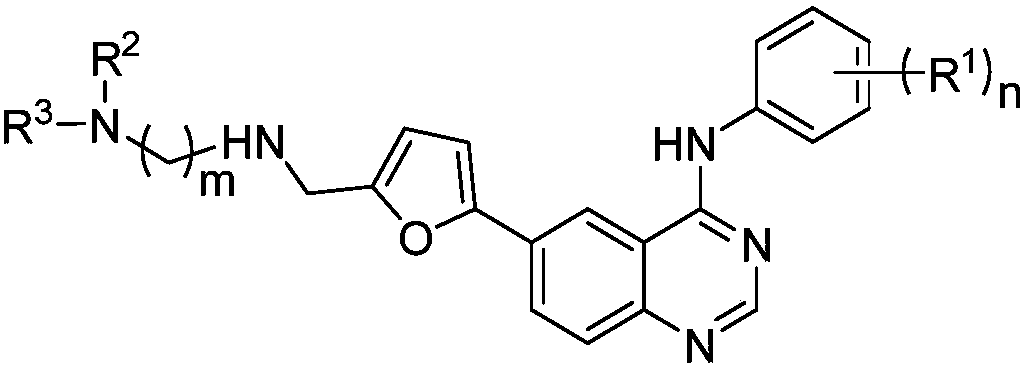
Dialkyl amino quinazoline compound and application thereof to preparation of anti-tumor medicines
A technology of dialkylaminoquinazoline and alkylaminoquinazoline is applied in the field of preparing antitumor drugs and can solve problems such as serious side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
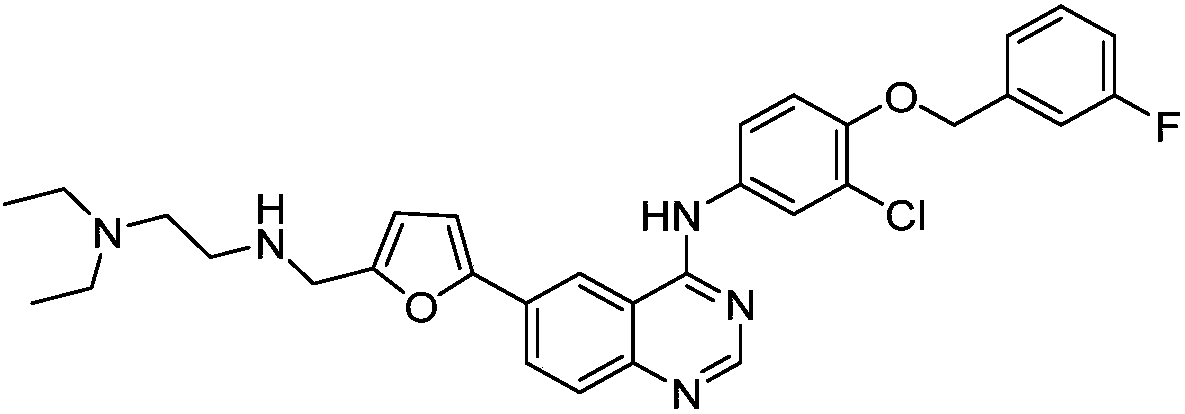
[0024] Synthetic compound 1: 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-[5-((N,N-diethylaminoethyl)aminomethyl)furan-2- base] quinazoline
[0025]
[0026] Add 0.09g (0.75mmol) of N,N-diethylaminoethylamine represented by formula II-1 and 5.0mL of methanol into a 50mL single-necked bottle, adjust the pH to 5-6 with formic acid, and then add 0.28g (2.00mmol ) anhydrous sodium sulfate, 0.06g (1.00mmol) sodium cyanoborohydride, under stirring, 4-[3-chloro-4-(3-fluorobenzyloxy) shown in 0.24g (0.50mmol) formula I-1 )anilino]-6-(5-formylfuran-2-yl)quinazoline and the mixture of 5mL tetrahydrofuran were dripped into the reaction system, after the dropwise addition, stirred at room temperature for 30 minutes, then added 0.06g (1.00 mmol) sodium cyanoborohydride, continue to stir and react for 90 minutes, adjust the pH to 9-10 with aqueous sodium hydroxide solution, filter with suction, evaporate the filtrate to remove the solvent, and separate the residue by silica gel column ch...
Embodiment 2
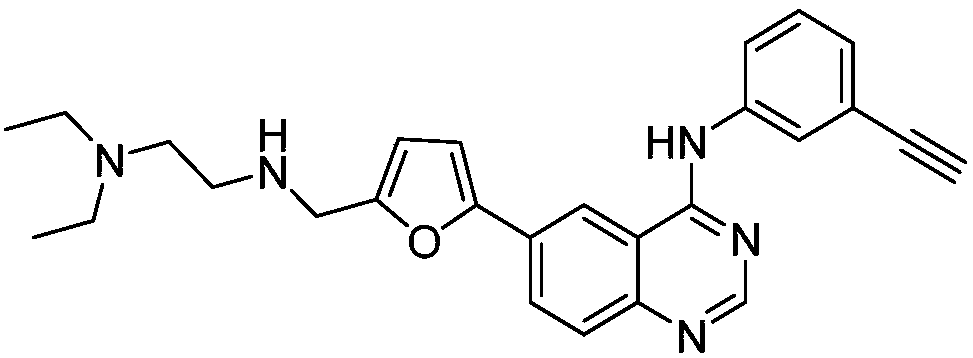
[0028] Synthetic compound 2: 4-(3-ethynylanilino)-6-[5-((N,N-diethylaminoethyl)aminomethyl)furan-2-yl]quinazoline
[0029]
[0030] Add 0.09g (0.75mmol) of N,N-diethylaminoethylamine represented by formula II-1 and 5.0mL of methanol into a 50mL single-necked bottle, adjust the pH to 5-6 with formic acid, and then add 0.28g (2.00mmol ) anhydrous sodium sulfate, 0.06g (1.00mmol) sodium cyanoborohydride, under stirring, 4-(3-ethynylphenylamino)-6-(5- The mixture of formylfuran-2-yl)quinazoline and 5mL tetrahydrofuran was dropped into the reaction system, after the dropwise addition, the reaction was stirred at room temperature for 30 minutes, then 0.06g (1.00mmol) sodium cyanoborohydride was added, and the stirring was continued React for 90 minutes, adjust the pH to 9-10 with aqueous sodium hydroxide solution, filter with suction, evaporate the filtrate to remove the solvent, and separate the residue by silica gel column chromatography (methanol:chloroform=1:15, V / V) to obtai...
Embodiment 3
[0032] Synthetic compound 3: 4-[4-(E)-propenylanilino]-6-[5-((N,N-diethylaminoethyl)aminomethyl)furan-2-yl]quinazoline
[0033]
[0034] Add 0.09g (0.75mmol) of N,N-diethylaminoethylamine represented by formula II-1 and 5.0mL of methanol into a 50mL single-necked bottle, adjust the pH to 5-6 with formic acid, and then add 0.28g (2.00mmol ) anhydrous sodium sulfate, 0.06g (1.00mmol) sodium cyanoborohydride, under stirring, 4-[4-(E)-propenylanilino]-6 shown in 0.18g (0.50mmol) formula I-3 The mixture of -(5-formylfuran-2-yl)quinazoline and 5mL tetrahydrofuran was dropped into the reaction system. After the dropwise addition, the reaction was stirred at room temperature for 30 minutes, and then 0.06g (1.00mmol) cyanoborohydrogenation was added Na, continue to stir and react for 90 minutes, adjust the pH to 9-10 with aqueous sodium hydroxide solution, filter with suction, evaporate the filtrate to remove the solvent, and separate the residue by silica gel column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com