Preparation method and application for 2-hydroxyl-4-alkoxy benzaldoxime
A technology of alkoxybenzaldehyde oxime and dihydroxybenzaldehyde, which is applied in the field of hydrometallurgical extractant and its preparation, can solve the problems of high cost, harsh synthesis conditions, and high synthesis difficulty, and achieve the improvement of copper-iron separation coefficient, The effect of reducing the difficulty of synthesis and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
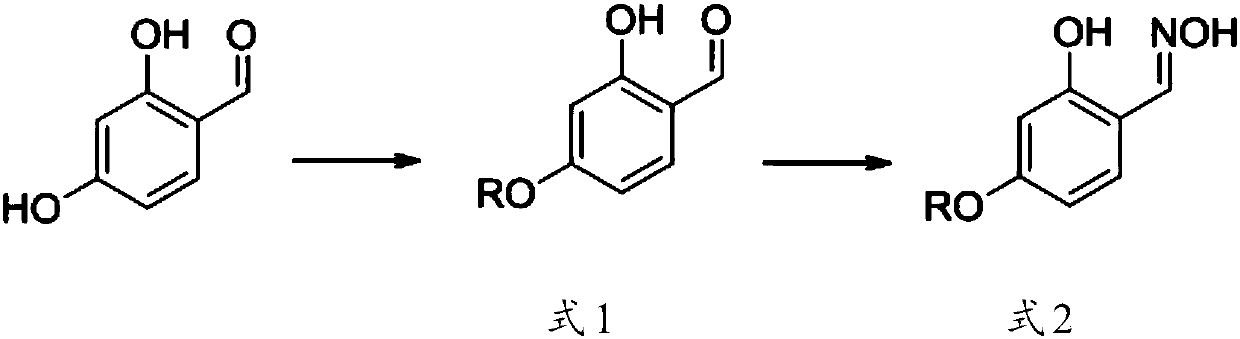
[0033] Preparation of copper extractant 2-hydroxyl-4-hexyloxybenzaldehyde oxime (in formula 2, R is C6)
example A
[0035] In this example, 10.0 g of 2,4-dihydroxybenzaldehyde was dissolved in 100 mL of acetone, 7.5 g of potassium bicarbonate (alkali) and 0.1 g of sodium iodide (catalyst) were added, and the reflux reaction was carried out for 1 hour, and then 12.1 g of bromine Hexane was dissolved in 20 mL of acetone and slowly added dropwise to the reaction solution. After 32 hours of reaction, 10 mL of hydroxylamine hydrochloride (5.0 g) and sodium acetate trihydrate (10.0 g) in water were added, stirred at room temperature for 1 hour to complete the reaction, and excess water was added. It was extracted with ethyl acetate, and the solvent was removed under reduced pressure to obtain a dark semi-solid crude product, which was separated by silica gel chromatography (eluent ethyl acetate:petroleum ether=1:6) to obtain 2-hydroxy-4-hexyloxybenzaldehyde The yield of pure oxime is 74%.
[0036] The proton nuclear magnetic resonance spectrum data of above-mentioned 2-hydroxyl-4-hexyloxybenzalde...
example B
[0039] The difference between this example and Example A is: the solvent for dissolving 2,4-dihydroxybenzaldehyde is acetone, the alkali added is potassium carbonate, and the catalyst is potassium iodide. The base added was potassium carbonate, stirred at room temperature until the reaction was completed, and subsequent extraction and purification were performed as in Example A to obtain pure 2-hydroxy-4-hexyloxybenzaldehyde oxime with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com