A drug support and preparation method thereof
A technology on drugs and stents, which is applied in the field of drug stents and its preparation, can solve problems such as vascular restenosis, achieve the effects of delaying endothelial repair, reducing endothelial cell growth inhibition, and shortening the time of antithrombotic and platelet drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
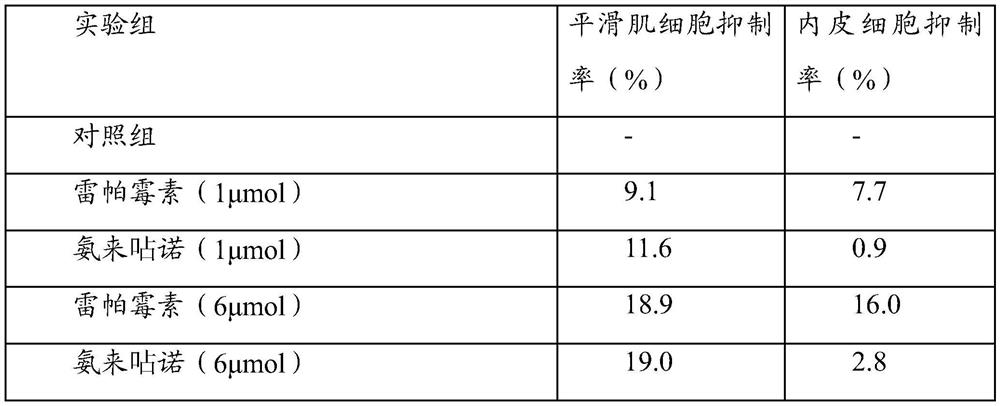
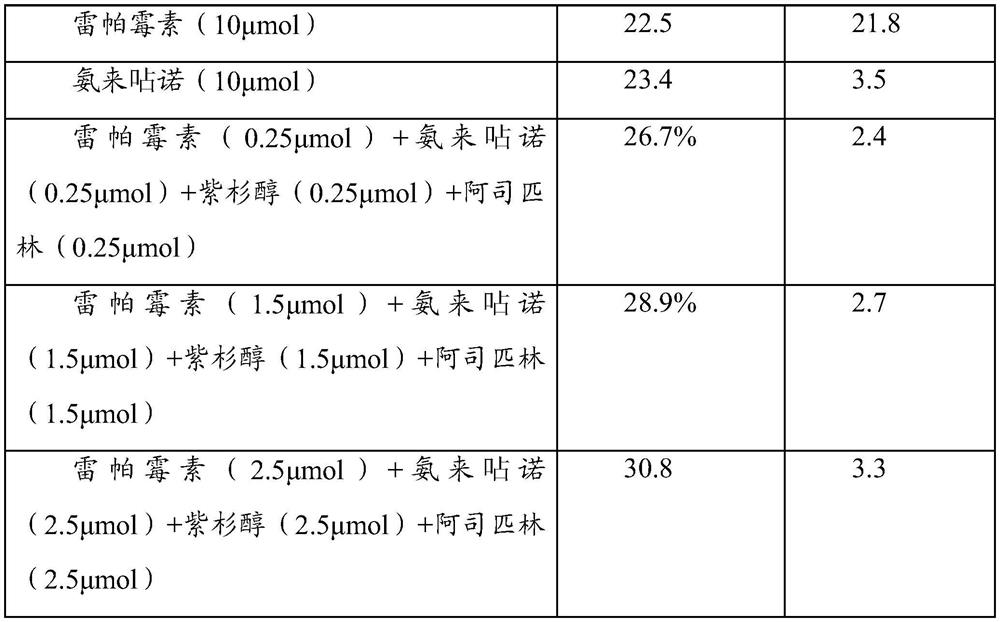
[0035] Experimental Example 1: Cell Experiment
[0036] A drug preparation:
[0037] (1) Amlexanox group:
[0038] a) Before the experiment, the drug stent loaded with amlexanox was placed in DMSO solution, and after it was completely dissolved, it was further diluted to form a drug concentration of 10 -2 mmol / ml solution.
[0039] b) add 10 -2 The drug solution of mmol / ml was diluted with dimethyl sulfoxide (DMSO) to a concentration of 10 -3 ,10 -4 ,10 -5 , 10 -6 ,10 -7 ,10 -8 ,10 -9 mmol / ml drug solution.
[0040] c) After dispensing the series of concentration drug solutions prepared above, store them temporarily at -20 degrees Celsius for later use.
[0041] d) When in use, dilute the stored DMSO for 10 -2 ~10 -9 After the amlexanox was taken out and returned to normal temperature, the drug solution of each concentration was diluted 1000 times with the corresponding complete cell culture medium to carry out the cell test, that is, the final use concentration w...
Embodiment 2
[0060] Embodiment 2 Carrier drug stent
[0061] Mix 50mg of amlexanox, 50mg of paclitaxel, 50mg of sirolimus, 50mg of aspirin and 500mg of PLGA in 100ml of acetone. After the solute is completely dissolved, spray the solution evenly on the L605 cobalt-chromium alloy metal stent by ultrasonic atomization On the surface of the body, the total drug loading of the four drugs reaches 130μg / cm 2 . The drug stent is prepared after the solvent is completely evaporated at room temperature.
Embodiment 3
[0062] Embodiment 3 Carrier-free drug stent
[0063] Fine lines are formed on the surface of the stainless steel stent body through friction treatment, and the micronized amlexanox, paclitaxel, sirolimus, and aspirin are placed in a high-pressure airtight place with the stent body at a weight ratio of 2:1:1:1. In the equipment, turn on the equipment, so that the drug particles are embedded in the fine lines of the stent body, and the total drug loading amount reaches 160μg / cm 2 , the average particle diameter of the drug particles is not more than 1 micron, and the drug stent is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com