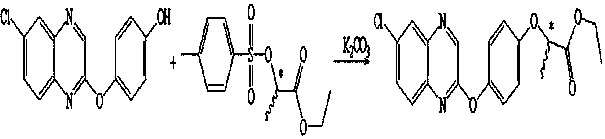
Quizalofop-p-ethyl as well as synthetic method of intermediate 4-(6-chloro-2 quinoxaline)phenol
A quinoxalineoxy and synthetic method technology, applied in the field of pesticide synthesis, can solve the problems of complex steps, low etherification product content, high alkali concentration, etc., and achieves the effects of reducing the generation of impurities, improving the utilization rate, and being easy to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 11.6g (0.105mol) of hydroquinone and 4.3g (0.105mol) of sodium hydroxide under nitrogen protection, add 60g of toluene and raise the temperature to 85°C for 0.5h, cool down and transfer to a constant pressure dropping funnel for later use. Add 20.2g (0.1mol) 2,6-dichloroquinoxaline and 220g toluene under the protection of nitrogen, raise the temperature to 100°C, add the prepared sodium salt of hydroquinone dropwise, finish dropping for 1 hour, and keep warm for 6 hours after dropping , sampling follow-up, the content of 2,6-dichloroquinoxaline is less than 0.5%, and after passing the pass, cool down to room temperature, filter and dry the filter cake, wash it with hot water until neutral to obtain 4-(6-chloro-2-quinoxaline oxygen Base) phenol 25.3g, content 96.2%, yield 89.3%.
[0024] Add 0.1mol of 4-(6-chloro-2quinoxalineoxy)phenol synthesized under nitrogen protection, 21.6g (0.156mol) of potassium carbonate, add 710g of non-polar solvent, heat up to anhydrous...
Embodiment 2
[0026] Weigh 14.3g (0.13mol) of hydroquinone and 4.7g (0.115mol) of sodium hydroxide under the protection of nitrogen, add 72g of toluene and raise the temperature to 85°C for 0.5h, cool down and transfer to a constant pressure dropping funnel for later use. Add 20.2g (0.1mol) 2,6-dichloroquinoxaline and 220g toluene under the protection of nitrogen, raise the temperature to 100°C, add the prepared sodium salt of hydroquinone dropwise, finish dropping for 1 hour, and keep warm for 6 hours after dropping , sampling follow-up, the content of 2,6-dichloroquinoxaline is less than 0.5%, and after passing the pass, cool down to room temperature, filter and dry the filter cake, wash it with hot water until neutral to obtain 4-(6-chloro-2-quinoxaline oxygen Base) phenol 25.7g, content 98.5%, yield 92.8%.
[0027] Add 0.1mol of 4-(6-chloro-2-quinoxalineoxy)phenol synthesized under nitrogen protection, 21.6g of potassium carbonate, add 730g of non-polar solvent, and add p-toluenesulfoni...
Embodiment 3
[0029] Weigh 12.7g (0.115mol) of hydroquinone and 4.3g (0.105mol) of sodium hydroxide under nitrogen protection, add 75g of toluene and raise the temperature to 85°C for 0.5h, cool down and transfer to a constant pressure dropping funnel for later use. Add 20.2g (0.1mol) 2,6-dichloroquinoxaline and 250g toluene under nitrogen protection, heat up to 100°C, add the prepared sodium salt of hydroquinone dropwise, finish dropping for 1 hour, and keep warm for 6 hours after dropping , sampling follow-up, the content of 2,6-dichloroquinoxaline is less than 0.5%, and after passing the pass, cool down to room temperature, filter and dry the filter cake, wash it with hot water until neutral to obtain 4-(6-chloro-2-quinoxaline oxygen Base) phenol 25.8g, content 98.8%, yield 93.5%.
[0030] Add 0.1mol of the 4-(6-chloro-2-quinoxalineoxy)phenol synthesized above under nitrogen protection, 21.6g of potassium carbonate, add 700g of non-polar solvent, and add p-toluenesulfonic acid lactic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com