Application of a blood-derived enzymatic peptide in the preparation of drugs for inhibiting intestinal inflammation in animals
A technology of intestinal inflammation and blood-derived enzymes, applied in the field of biomedicine, can solve problems such as exacerbating drug residues, carcinogenesis, and human harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
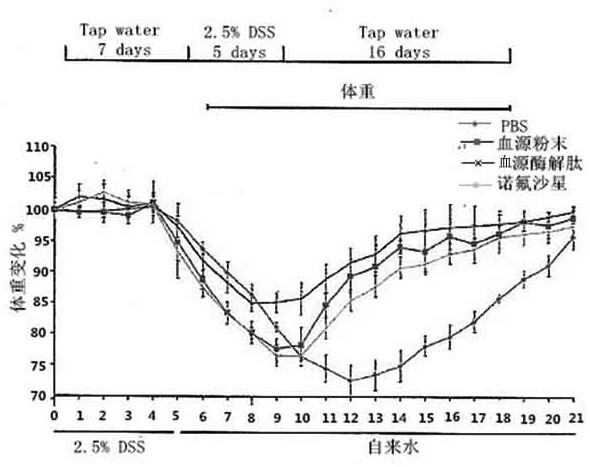
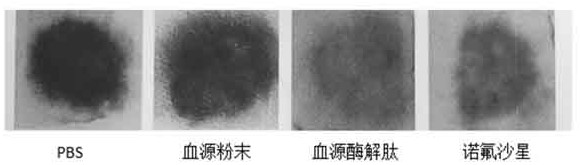
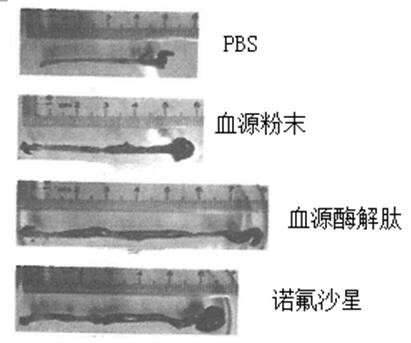
[0024] 5.0 g of pig blood, 0.15 mg of trypsin, 0.15 mg of gastric protease, 40ml of phosphoric acid buffered solution in the cone bottle, enzymatic solution in the shaker. The shaker is set to 37 degrees, and the stirring speed is 150R / MIN, the enzymatic solution is 6 hours. The enzymatic hydrolyzate centrifugal was centrifuged, and the rotational speed of the centrifuge was set to 5000 rpm / min, and the centrifugal time was 10 min. Deeply precipitate, take it, repeat the centrifugation twice; the resulting filtrate vacuum filtrate, the resulting filtrate was filtered, the resulting filtrate was filtered twice, the enzymatic epoxine was filtered twice, and the ultrafiltration was removed (the pore diameter of 3000 Da) was 2500 rpm. / min centrifugally filtered 3000 Da or less filtration, and then lyophilized in a vacuum freeze dryer, and the blood source enzyme peptide powder was obtained. The acute intestinal inflammatory model of C57BL / 6 mice was established using DSS: usin...
Embodiment 2
[0026] 5.0 g of pig blood, 0.15 mg of trypsin, 0.15 mg of gastric protease, 40ml of phosphoric acid buffered solution in the cone bottle, enzymatic solution in the shaker. The shaker is set to 37 degrees, and the stirring speed is 150R / MIN, the enzymatic solution is 6 hours. The enzymatic hydrolyzate centrifugal was centrifuged, and the rotational speed of the centrifuge was set to 5000 rpm / min, and the centrifugal time was 10 min. Deeply precipitate, take it, repeat the centrifugation twice; the resulting filtrate vacuum filtrate, the resulting filtrate was filtered, the resulting filtrate was filtered twice, the enzymatic epoxine was filtered twice, and the ultrafiltration was removed (the pore diameter of 3000 Da) was 2500 rpm. / min centrifugally filtered 3000 Da or less filtration, and then lyophilized in a vacuum freeze dryer, and the blood source enzyme peptide powder was obtained. The acute intestinal inflammatory model of C57BL / 6 mice was established using DSS: usin...
Embodiment 3
[0039]5.0 g of pig blood, 0.15 mg of trypsin, 0.15 mg of gastric protease, 40ml of phosphoric acid buffered solution in the cone bottle, enzymatic solution in the shaker. The shaker is set to 37 degrees, and the stirring speed is 150R / MIN, the enzymatic solution is 6 hours. The enzymatic hydrolyzate centrifugal was centrifuged, and the rotational speed of the centrifuge was set to 5000 rpm / min, and the centrifugal time was 10 min. Deeply precipitate, take it, repeat the centrifugation twice; the resulting filtrate vacuum filtrate, the resulting filtrate was filtered, the resulting filtrate was filtered twice, the enzymatic epoxine was filtered twice, and the ultrafiltration was removed (the pore diameter of 3000 Da) was 2500 rpm. / min centrifugally filtered 3000 Da or less filtration, and then lyophilized in a vacuum freeze dryer, and the blood source enzyme peptide powder was obtained. The acute intestinal inflammatory model of C57BL / 6 mice was established using DSS: using...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com