Desvenlafaxine succinate impurities as well as preparation method and use thereof
A kind of technology of desvenlafaxine succinate and impurities, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
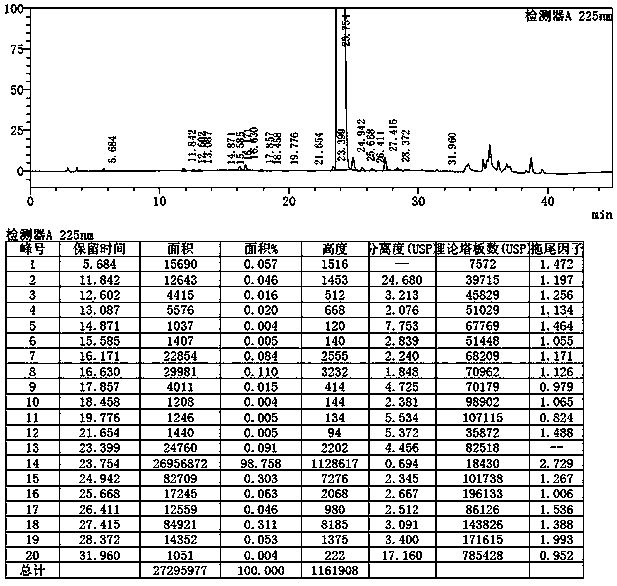
[0086] The preparation of example 1 desvenlafaxine succinate impurity 2
[0087]
[0088] 1. Preparation of impurities 2
[0089] Add (5.00g (0.018mol) 1-(2-amino-1-(4-methoxyphenyl)ethyl)cyclohexanol hydrochloride (intermediate B) and 50ml toluene into a three-necked flask, stir to dissolve After clearing, add 1.88g triethylamine (0.15mol) and stir for 8-10 minutes, add 10.65g p-toluenesulfonic acid (0.061mol), stir and heat up to 100-110°C, keep warm for 3-5h. After the reaction, cool down to 5~15°C, add 10% sodium hydroxide solution to adjust the pH=9~10, separate the liquids, extract the organic phase twice with 25ml ethyl acetate, combine the organic phases, add 10g anhydrous sodium sulfate to dry and dehydrate, filter, and collect Concentrate the filtrate under reduced pressure, add 10ml of isopropanol and 10ml of isopropyl ether to the residue, stir to dissolve, cool down to 0~10°C, add hydrochloric acid ethanol dropwise to adjust the pH to 2~3, stir to crystallize,...
example 2
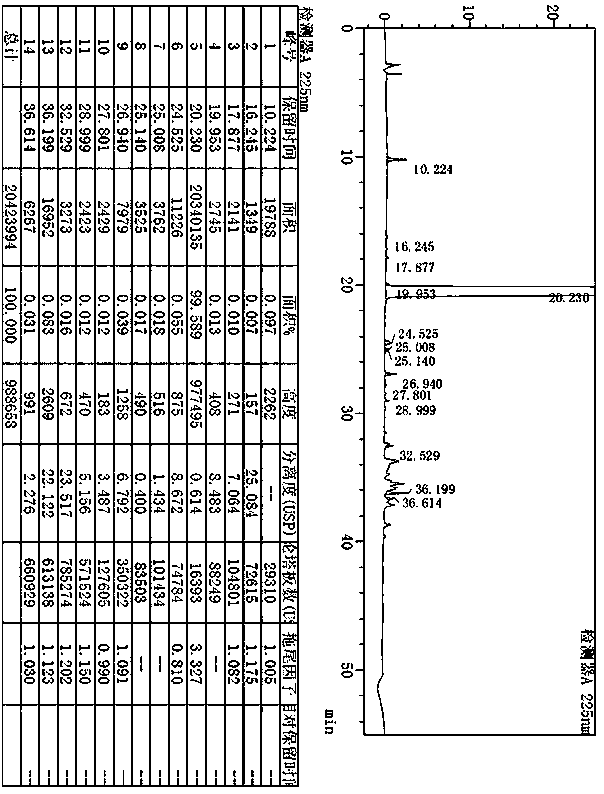
[0091] The preparation of example 2 desvenlafaxine succinate impurity 5
[0092]
[0093] Add 5.00g (17mmol) of 2-cyclohexyl-2-(4-methoxyphenyl)-N,N-dimethylethylamine hydrochloride (impurity G) to the reaction flask, dodecyl Sodium thiol 15.00g (59mmol), N-methylpyrrolidone 30ml, stir and heat up to 170~180℃, keep warm for 5~8h. After the reaction is complete, cool down to below 40°C, add 20ml of water and 20ml of ethyl acetate, cool down to 0~10°C, add concentrated hydrochloric acid dropwise to adjust the pH to 1~2, separate the liquids, extract the aqueous phase with 20ml of ethyl acetate, and collect For the water phase, cool down to 0~10°C, adjust the pH to 9~10 with 10% sodium hydroxide solution, filter, collect the filter cake, stir in 10ml of ethyl acetate and heat up to 7~80°C, stir to dissolve, then cool down Stir to crystallize at 5~15°C, filter, and dry the filter cake under reduced pressure to obtain the target compound 4-(1-cyclohexanol-2-(N,N-dimethylamino)e...
example 3
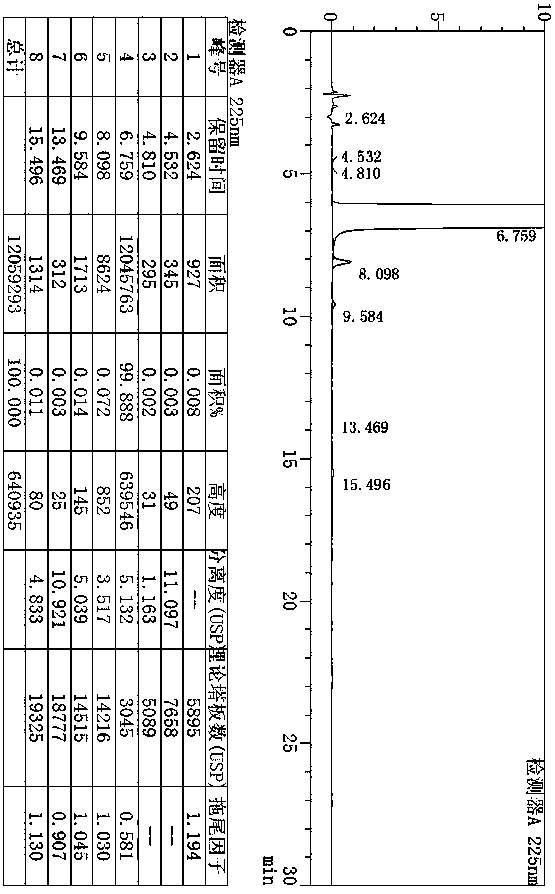
[0094] The preparation of example 3 desvenlafaxine succinate impurity 6
[0095]
[0096] Add 8.00g (37mmol) of 2-(4-methoxyphenyl)-N,N-dimethylethylammonium hydrochloride and 80ml (40%) of hydrobromic acid into the reaction flask, stir and raise the temperature to 120~130 ℃ for 3~5 hours. After the reaction is complete, lower the temperature to 0~20°C, add dropwise 40% sodium hydroxide solution to adjust the pH to 9~11, add 24ml of ethyl acetate to stir and separate the liquid, extract the aqueous phase with 24ml of ethyl acetate, combine the organic phases, and add 12g Dry and dehydrate with anhydrous sodium sulfate, filter, collect the filtrate and concentrate it to dryness under reduced pressure, then add 8ml of ethyl acetate to the residue and stir to raise the temperature to 65~75°C. The cake was dried under reduced pressure to obtain 4.35 g of the target compound 4-(2-(N,N-dimethylamino)ethyl)phenol (impurity 6), with a yield of 71.54% and a purity of 98.64%. h 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com