Preparation method of 2-(3-amino-4-chlorobenzoyl)benzoic acid
A technology of chlorobenzoyl and nitrobenzoyl, which is applied in the field of preparation of 2-benzoic acid, can solve the problems of unsuitability for industrial production, unsafe process, long reaction time, etc., and achieve low cost, high purity, and reduced Effects of Response Costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of 2-(3-amino-4-chlorobenzoyl)benzoic acid, comprising the following steps:
[0025] Mix 2-(4-chloro-3-nitrobenzoyl)benzoic acid, Raney nickel and an organic solvent, and carry out a hydrogenation reaction under a hydrogen atmosphere to obtain 2-(3-amino-4-chlorobenzoyl )benzoic acid;
[0026] The time for the hydrogenation reaction is 1 to 5 hours.
[0027] In the present invention, the raw materials used in the preparation method can all be commercially available raw materials well known to those skilled in the art, and will not be described in detail below.
[0028] The invention mixes 2-(4-chloro-3-nitrobenzoyl)benzoic acid, Raney nickel and an organic solvent to obtain a raw material mixture. The present invention has no special requirements on the mixing method, as long as all components can be mixed evenly. The present invention uses commercially available Raney nickel, and the Raney nickel product contains 2...
Embodiment 1
[0047]Add 100 g (0.33 mol) of 2-(3-nitro-4-chlorobenzoyl)benzoic acid, 600 g of ethyl acetate, and 10 g of Raney nickel (20% water content) into a 1L stainless steel autoclave, and seal the kettle after adding , start stirring, nitrogen replacement three times, pass hydrogen to 1.0MPa, heat up to 35°C for reaction, when the hydrogen pressure is 98% detected by HPLC, yield 95%, melting point 179~183°C.
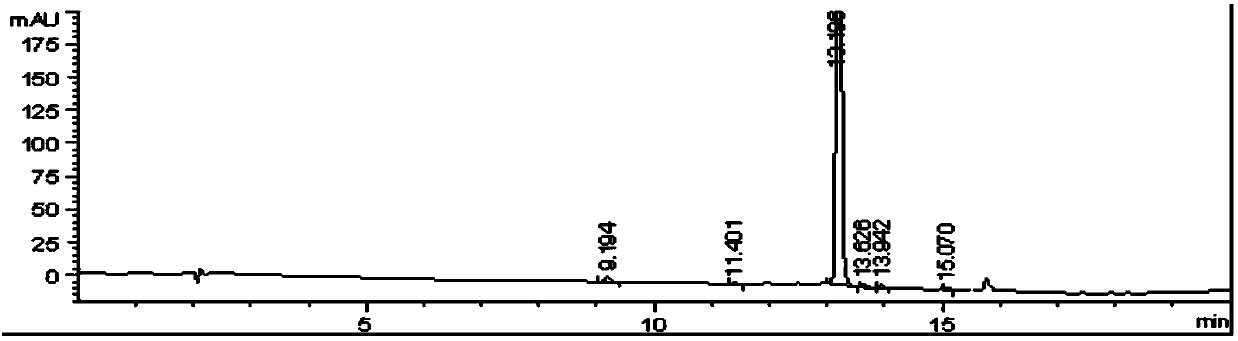
[0048] The HPLC detection result of described 2-(3-amino-4-chlorobenzoyl) benzoic acid is as table 1 and figure 1 shown.
[0049] The HPLC detection result of 2-(3-amino-4-chlorobenzoyl)benzoic acid in the embodiment 1 of table 1
[0050]
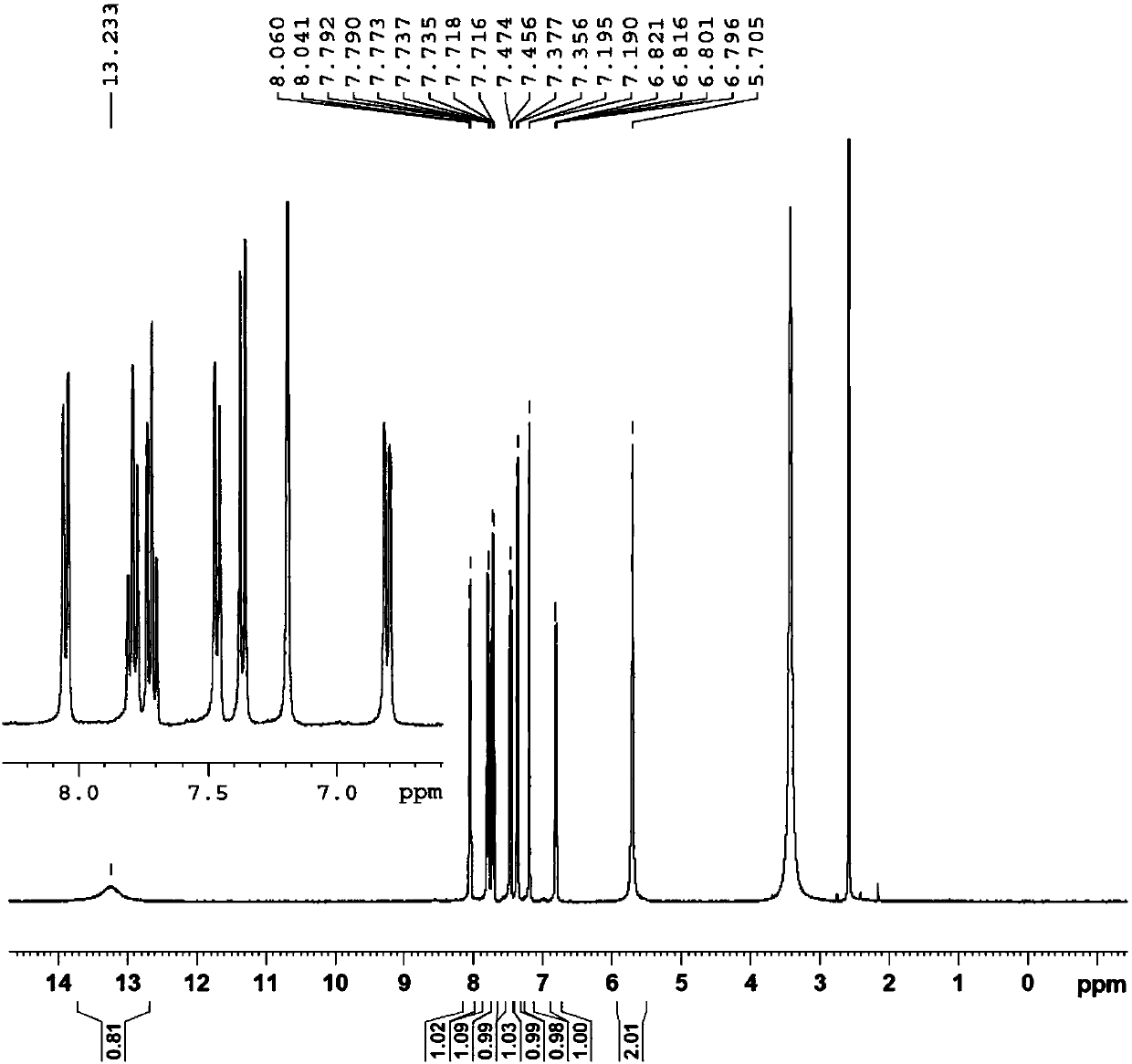
[0051] The structure of the product was identified using nuclear magnetic resonance, and the results were as follows figure 2 shown, combined with figure 2 It can be seen that the obtained identification data are as follows:
[0052] 1 H NMR (DMSO-d6,400MHz)δ13.233(s,1H),8.041-8.060(d,1H),7.773-7.792(t,1H),7.716-7.737(m,1H),7.456-7...
Embodiment 2
[0054] In 1L stainless steel autoclave, add 2-(3-nitro-4-chlorobenzoyl) benzoic acid 100g (0.33mol), ethyl acetate 600g, the Raney nickel 10g (water content 20%) that embodiment 1 obtains and fresh Raney nickel 2g (water content 20%), after the addition, seal the kettle, start stirring, replace with nitrogen three times, pass hydrogen to the pressure of 2.0MPa, heat up to 40°C for reaction, when the hydrogen pressure is 98% detected by HPLC, yield 96%, melting point 179~183°C.
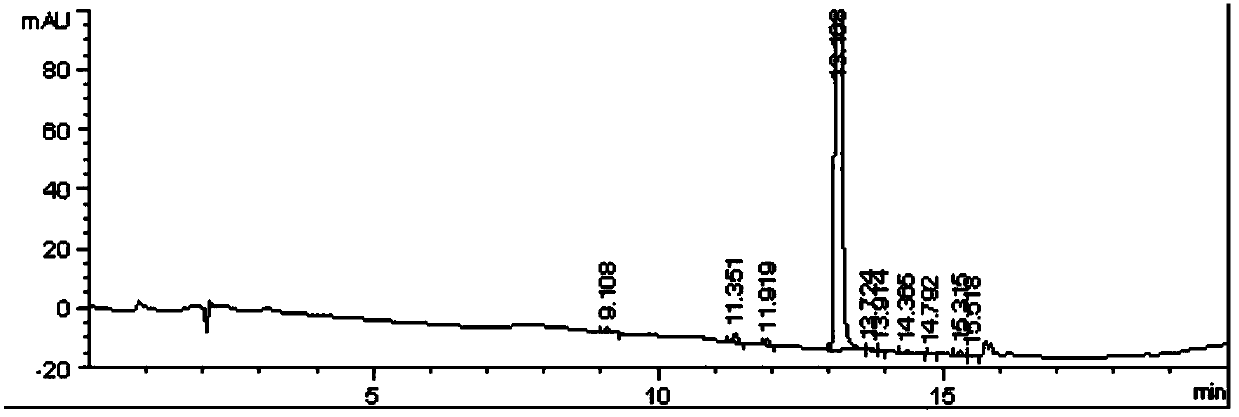
[0055] The HPLC detection result of described 2-(3-amino-4-chlorobenzoyl) benzoic acid is as table 2 and image 3 shown.
[0056] The HPLC detection result of 2-(3-amino-4-chlorobenzoyl)benzoic acid in the embodiment 2 of table 2
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com