Preparation method of energetic compound 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide
A technology of triaminopyrimidine and nitropyrimidine, which is applied in the field of energetic material synthesis, can solve the problems affecting the miniaturization process of combat damage parts and low energy, and achieve low cost of raw materials and solvents, short synthesis steps, and simple post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
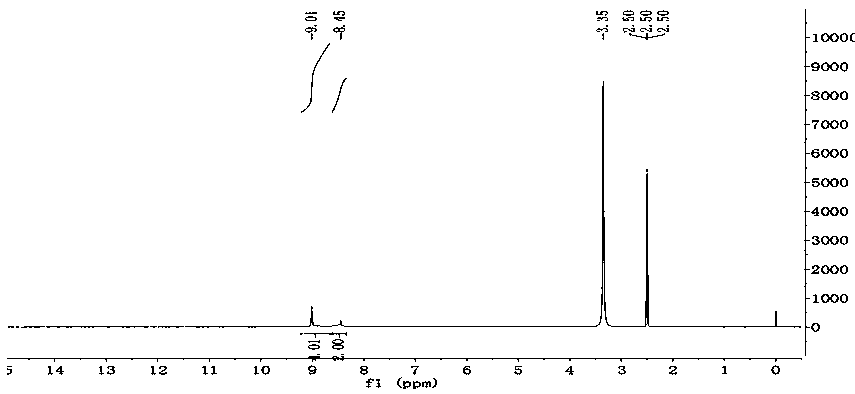
[0019] The structural formula of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide synthesized by the preparation method of the present invention is:
[0020]
[0021] The preparation method of the present invention adopts following three synthetic routes:
[0022] Route 1: Using 2,4,6-triaminopyrimidine as the reaction raw material, the energetic compound 2,4,6-triamino-5-nitropyrimidine-1,3- dioxide.
[0023] Under the condition of temperature -10~50℃, slowly add 2,4,6-triaminopyrimidine in batches to a certain amount of nitration reagent, the nitration reagent used includes 90%~100% nitric acid, Nitric acid / sulfuric acid mixture, nitric acid / acetic acid mixture, nitric acid / trifluoroacetic acid mixture, nitric acid / trifluoromethanesulfonic acid mixture, nitric acid / acetic anhydride mixture, nitric acid / trifluoroacetic anhydride mixture, or tetrafluoroboric acid One of the nitroxyl reagents; continue to react for 1 to 24 hours after the addition, then cool the reaction syste...
Embodiment 1
[0031] Preparation of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide (Route 1)
[0032] (1) At -10°C, slowly add 8ml of fuming nitric acid dropwise into 8ml of concentrated sulfuric acid, and add 2g (16mmol) of 2,4,6-triaminopyrimidine to the above mixed acid several times in batches. After the addition, The reaction system was reacted at this temperature for 5 hours, the reaction liquid was poured into ice water, the solid was suction filtered and dried to obtain 2,4,6-triamino-5-nitropyrimidine with a yield of 90%.
[0033] (2) Dissolve 1 g (5.9 mmol) of 2,4,6-triamino-5-nitropyrimidine in 5 ml of trifluoroacetic acid at -10°C and stir slowly, and slowly add 2.5 ml of aqueous hydrogen peroxide (over Hydrogen oxide mass fraction: 20%), after the dropwise addition, react at this temperature for 24 hours, and filter with suction to obtain a solid. The solid was dissolved in water, neutralized with saturated aqueous sodium carbonate solution to neutrality, and the solid was filte...
Embodiment 2
[0035] Preparation of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide (Route 1)
[0036] (1) At 0°C, in 20 ml of nitric acid with a mass fraction of 100%, 2 g (16 mmol) of 2,4,6-triaminopyrimidine was added to the above acid several times in batches. After the addition, the reaction system was React at this temperature for 4 hours, pour the reaction solution into ice water, filter the solid with suction, and dry to obtain 2,4,6-triamino-5-nitropyrimidine with a yield of 92%.
[0037] (2) Dissolve 1g (5.9 mmol) of 2,4,6-triamino-5-nitropyrimidine in 10 ml of concentrated sulfuric acid at 20°C and stir, slowly add 5 ml of hydrogen peroxide aqueous solution (hydrogen peroxide Mass fraction: 27%), after the dropwise addition, react at this temperature for 12 hours, and filter with suction to obtain a solid. The solid was dissolved in water, neutralized with saturated potassium carbonate aqueous solution to neutrality, and the solid was filtered with suction to obtain 2,4,6-triamino-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com