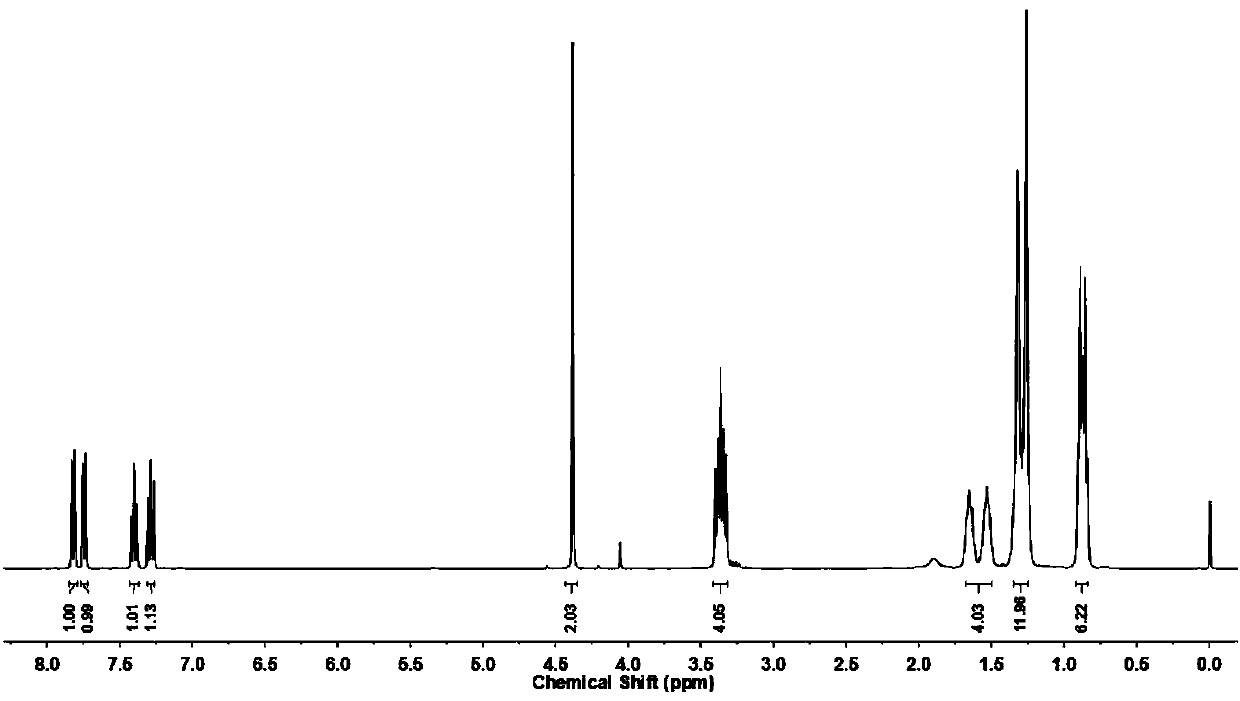
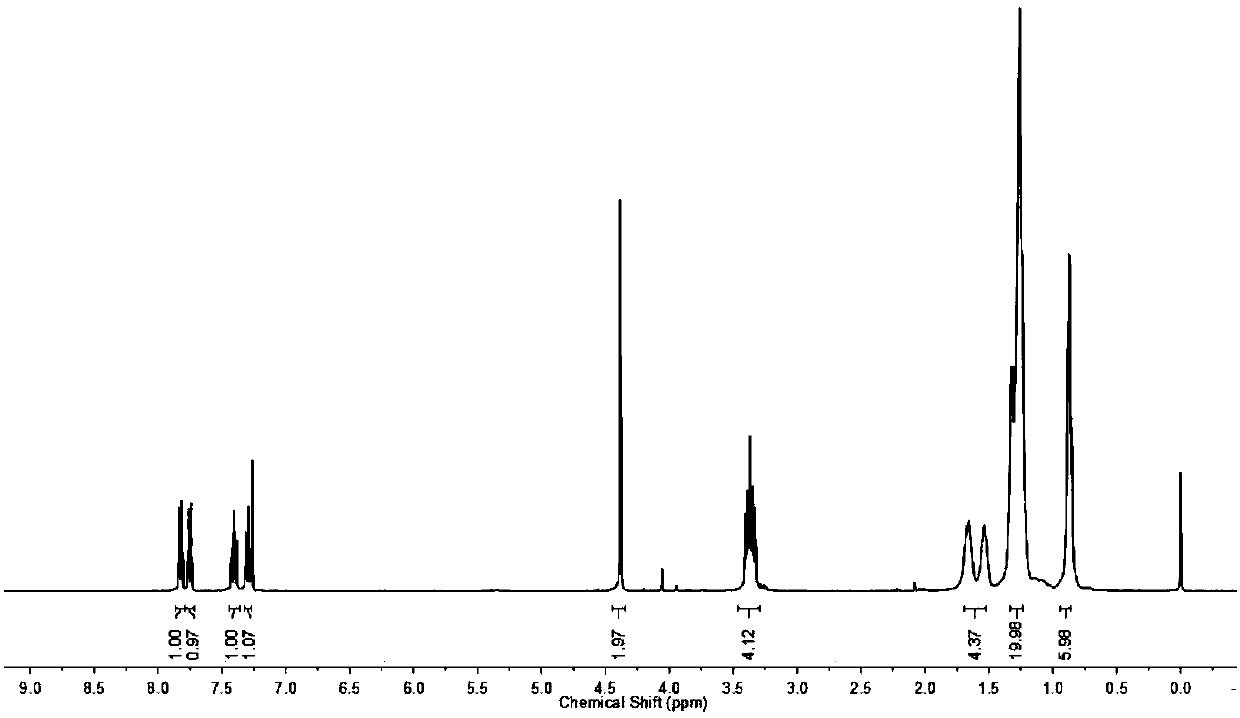
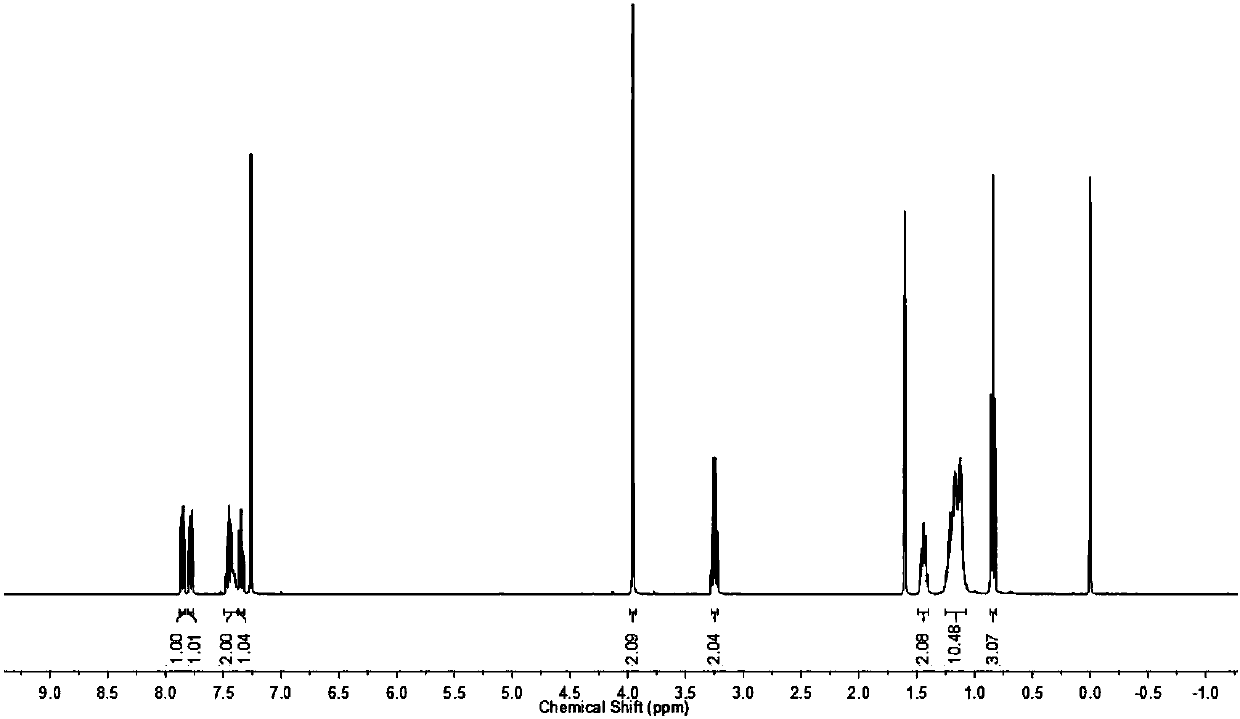
Benzothiazole derivative multifunctional lubricating oil additive, preparation method and application thereof
A lubricating oil additive, benzothiazole technology, applied in the field of benzothiazole derivative multifunctional lubricating oil additive, can solve the problems of poor oxidation resistance, electrochemical corrosion and the like, achieve high synthesis yield, mild reaction conditions, Excellent extreme pressure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 0.05mol (9.27g) of dihexylamine, 0.06mol (6.07g) of triethylamine and 80mL of chloroform were sequentially added to a 250mL three-necked flask, and 0.05mol (6.78g) of chloroacetyl chloride was added dropwise in an ice bath, and the reaction was stirred for 3.0h. After the reaction was completed, the reaction solution was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain N,N-dihexyl-2-chloroacetamide.
[0039] Add 0.05mol (8.36g) 2-mercaptobenzothiazole, a small amount of tetrabutylammonium bromide, 60mL 5.0% (mass fraction) NaOH aqueous solution and 30mL tetrahydrofuran to a 250mL three-necked flask, stir at room temperature until the solids are completely dissolved, and 0.06mol (4.57g) of carbon disulfide was added dropwise and reacted for 0.5h. The above-mentioned N,N-dihexyl-2-chloroacetamide was dissolved in a small amount of tetrahydrofuran and dropped into the above...
Embodiment 2
[0041] 0.05mol (12.08g) of dioctylamine, 0.06mol (6.07g) of triethylamine and 80mL of chloroform were sequentially added to a 250mL three-necked flask, and 0.05mol (6.78g) of chloroacetyl chloride was added dropwise in an ice bath, and the reaction was stirred for 3.0h. After the reaction was completed, the reaction liquid was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain N,N-dioctyl-2-chloroacetamide.
[0042] Add 0.05mol (8.36g) 2-mercaptobenzothiazole, a small amount of tetrabutylammonium bromide, 60mL 5.0% (mass fraction) NaOH aqueous solution and 30mL tetrahydrofuran to a 250mL three-necked flask, stir at room temperature until the solids are completely dissolved, and 0.06mol (4.57g) of carbon disulfide was added dropwise and reacted for 0.5h. The above-mentioned N,N-dioctyl-2-chloroacetamide was dissolved in a small amount of tetrahydrofuran and dropped into the above ...
Embodiment 3
[0044] 0.05mol (6.46g) of n-octylamine, 0.06mol (6.07g) of triethylamine and 80mL of chloroform were sequentially added to a 250mL three-necked flask, and 0.05mol (6.78g) of chloroacetyl chloride was added dropwise in an ice bath, and the reaction was stirred for 3.0h. After the reaction was completed, the reaction liquid was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain N-n-octyl-2-chloroacetamide.
[0045] Add 0.05mol (8.36g) 2-mercaptobenzothiazole, a small amount of tetrabutylammonium bromide, 60mL 5.0% (mass fraction) NaOH aqueous solution and 30mL tetrahydrofuran to a 250mL three-necked flask, stir at room temperature until the solids are completely dissolved, and 0.06mol (4.57g) of carbon disulfide was added dropwise and reacted for 0.5h. The above-mentioned N-n-octyl-2-chloroacetamide was dissolved in a small amount of tetrahydrofuran and then dropped into the above-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Onset thermal decomposition temperature | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com