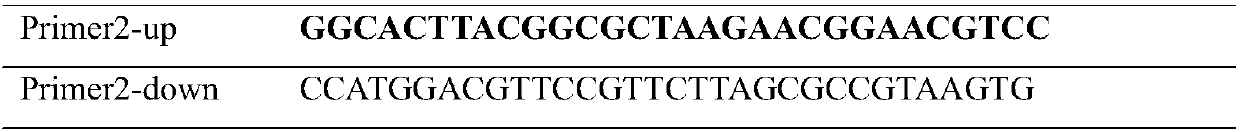Nattokinase with improved acid resistance
A nattokinase and amino acid technology, applied in the field of protein engineering, can solve the problems of industrial application of restriction enzymes, poor acid resistance, etc., and achieve the effects of good acid stability and improved acid resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The construction of embodiment 1 nattokinase single mutant and double mutant
[0025] (1) Construction of single mutant Q59E
[0026] Using pET24a-pro-NK as a template, and using the primers shown in Table 1 under the conditions shown in Table 5, a recombinant vector carrying the gene encoding the Q59E mutant was obtained by PCR to obtain the plasmid pET24a-pro-NK / Q59E.
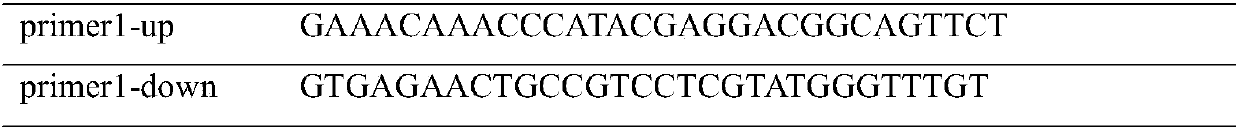
[0027] Table 1 Mutant Q59E primers
[0028]
[0029] The PCR products were analyzed and identified by 1.0% agarose gel electrophoresis; the PCR products were purified with a DNA purification kit, and finally were purified by QuichCut Tm DpnI was digested at 37°C for 5 hours to remove the methylated template plasmid, and then transformed into BL21 Escherichia coli host, and the recombinant strain was named BL21 / pET24a-pro-NK / Q59E.
[0030] (2) Construction of single mutant Y217K
[0031] Using pET24a-pro-NK as a template, using the primers shown in Table 2 and the conditions shown in Table 5, a r...
Embodiment 2
[0043] The construction of embodiment 2 nattokinase triple mutants
[0044]Using pET24a-pro-NK as a template, using the primers shown in Table 1 and the conditions shown in Table 5, PCR was performed to obtain a recombinant vector carrying the gene encoding the Q59E mutant; use the plasmid mini-extraction kit to extract pET24a-pro according to the instructions. -NK / Q59E plasmid, then using pET24a-pro-NK / Q59E as a template, and using the primers shown in Table 4 under the conditions shown in Table 5, PCR was obtained to carry the triple mutant gene encoding Q59E-Y217K-N218D, and the plasmid was named pET24a -pro-NK / Q59E-Y217K-N218D.
[0045] Table 4 mutant Q59E-Y217K-N218D primers
[0046]
[0047] Table 5 Whole plasmid PCR amplification reaction system
[0048]
[0049] The PCR amplification reaction conditions are:
[0050]
[0051] The PCR products were analyzed and identified by 1.0% agarose gel electrophoresis; the PCR products were purified with a DNA purifica...
Embodiment 3
[0052] Expression of embodiment 3 nattokinase mutants
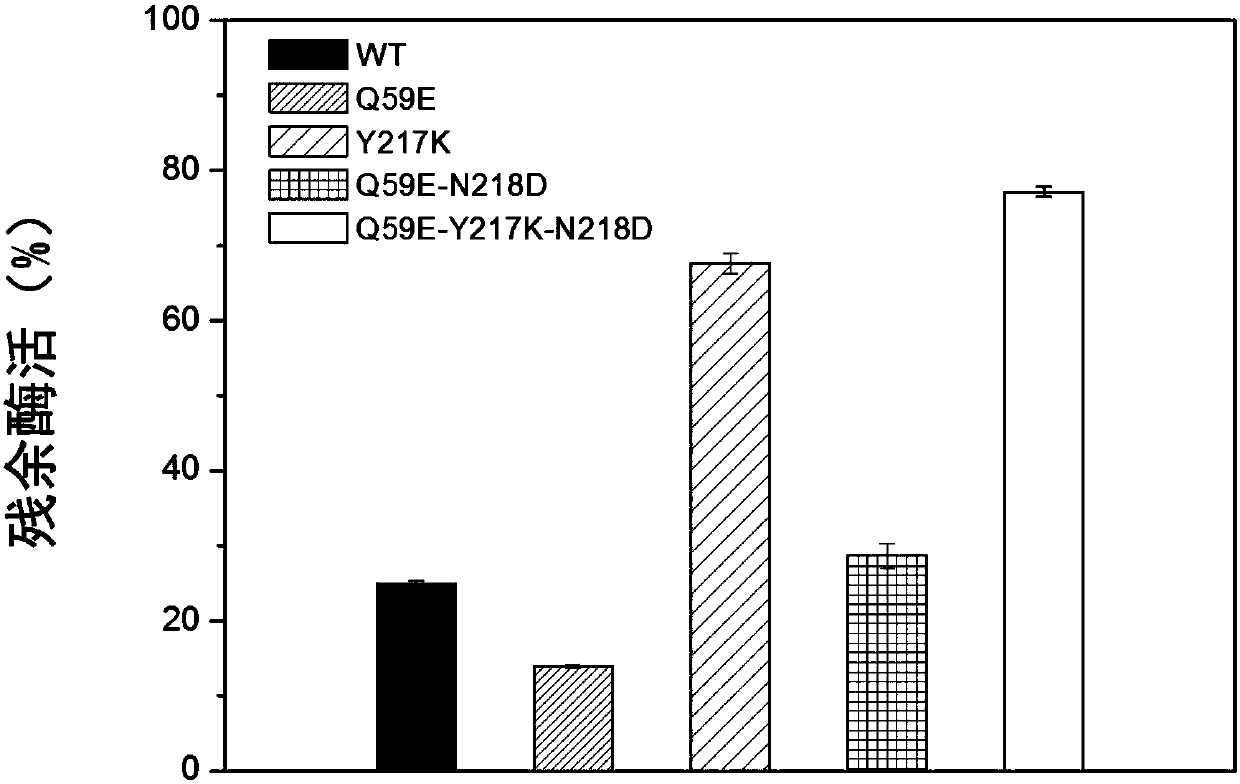
[0053] (1) Recombinant Escherichia coli BL21 / pET24a-pro-NK / Q59E-Y217K-N218D, BL21 / pET24a-pro-NK / Q59E, BL21 / pET24a-pro-NK / Y217K, BL21 / pET24a-pro-NK / N218D , BL21 / pET24a-pro-NK / Q59E were inoculated in 5 mL of LB medium with a kanamycin concentration of 100 μg / mL, cultured overnight at 37°C with shaking at 200 r / min.
[0054] (2) Inoculate the above seed liquid into 100 mL of TB expression medium containing 100 μg / mL of kanamycin at an inoculum amount of 1% by mass fraction, and culture it with shaking at 37 ° C and 200 r / min until OD 600 At 0.6-0.8, add the inducer IPTG to 0.1mM, induce for 20 hours at 18°C to collect the bacteria, and centrifuge at a speed of 5000g to collect the bacteria.
[0055] (3) Dissolve the recombinant bacteria in 20mL binding buffer solution (50mmol / LNa 2 HPO 4 , 50mmol / LNaH 2 PO 4 , 500mmol / LNaCl, 20mmol / L imidazole), ultrasonically crushed, centrifuged at 13000g for 25min, and the supernat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com