Lithium cobalt oxide-coated lithium nickel cobalt aluminate positive electrode material and preparation method thereof
A technology of nickel-cobalt-lithium aluminate and positive electrode materials, which can be applied to battery electrodes, electrical components, electrochemical generators, etc., and can solve the problems of affecting electrochemical performance, low price of nickel-cobalt lithium aluminate, uneven coating layer, etc. , to achieve good cycle stability and high rate discharge performance, reduce surface residual lithium, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A nickel-cobalt-lithium-aluminate cathode material coated with lithium cobaltate:
[0041] The mass percentage of the lithium cobaltate is 1wt%, and the lithium cobaltate forms a coating layer with a thickness of 8-15nm to cover the nickel-cobalt-aluminate lithium; the positive electrode material is a spherical particle with a particle size of 5-15μm.
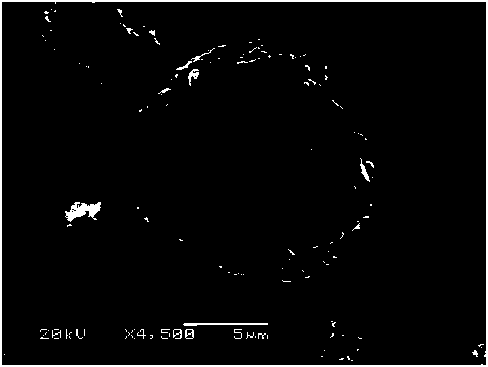
[0042] likefigure 1 As shown, the nickel-cobalt-lithium-aluminate cathode material coated with lithium cobaltate obtained in the embodiment of the present invention is a spherical particle with a particle size of 5-15 μm, with a lithium cobaltate coating layer on the surface.
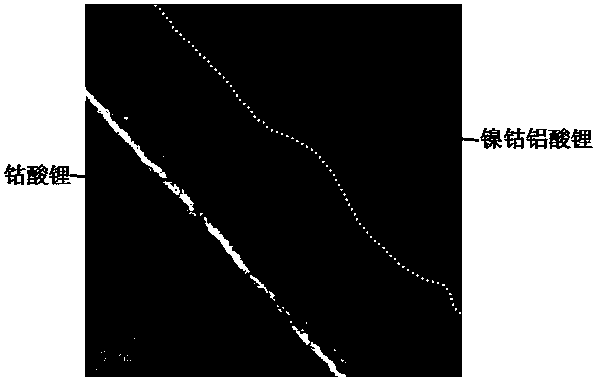
[0043] like figure 2 As shown, the matrix part of the lithium cobalt oxide-coated lithium nickel cobalt aluminate positive electrode material obtained in the embodiment of the present invention is lithium nickel cobalt aluminate, and a lithium cobalt oxide coating layer with a thickness of 8-15 nm is formed on the surface.
[0044] like image ...
Embodiment 2
[0055] A nickel-cobalt-lithium-aluminate cathode material coated with lithium cobaltate:
[0056] The mass percentage of the lithium cobaltate is 3wt%, and the lithium cobaltate forms a coating layer with a thickness of 10-20nm to cover the nickel-cobalt-aluminate lithium; the positive electrode material is a spherical particle with a particle size of 5-15 μm.
[0057] After testing, the nickel-cobalt-lithium-aluminate cathode material coated with lithium cobaltate obtained in the embodiment of the present invention is a spherical particle with a particle size of 5-15 μm, with a lithium cobaltate coating layer on the surface.
[0058] After testing, the matrix of the lithium cobalt oxide-coated lithium nickel cobalt aluminate positive electrode material obtained in the embodiment of the present invention is lithium nickel cobalt aluminate, and a lithium cobalt oxide coating layer with a thickness of 10-20 nm is formed on the surface.
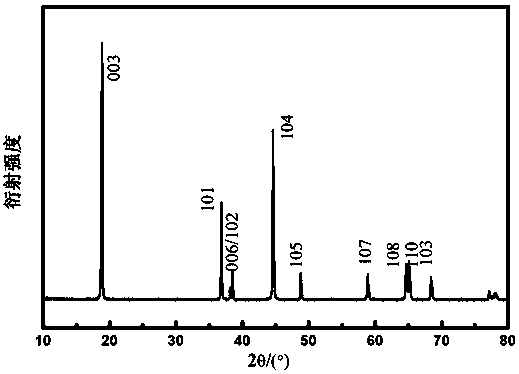
[0059] After testing, the 006 and 102, 10...
Embodiment 3
[0070] A nickel-cobalt-lithium-aluminate cathode material coated with lithium cobaltate:
[0071] The mass percentage of the lithium cobaltate is 4.8wt%, and the lithium cobaltate forms a coating layer with a thickness of 15-25nm in an amorphous state and coats the nickel-cobalt-aluminate lithium; the positive electrode material has a particle size of 5 ~15 μm spherical particles.
[0072] After testing, the nickel-cobalt-lithium-aluminate cathode material coated with lithium cobaltate obtained in the embodiment of the present invention is a spherical particle with a particle size of 5-15 μm, with a lithium cobaltate coating layer on the surface.
[0073] After testing, the matrix part of the lithium cobalt oxide-coated lithium nickel cobalt aluminate positive electrode material obtained in the embodiment of the present invention is lithium nickel cobalt aluminate, and a lithium cobalt oxide coating layer with a thickness of 15-25 nm is formed on the surface.
[0074] After t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com