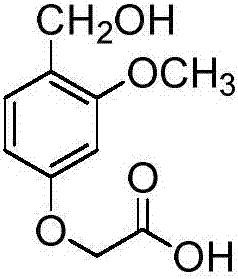
Preparation method of 4-hydroxymethyl-3-methoxyphenoxyacetic acid
A kind of technology of methoxyphenoxyacetic acid and methoxyphenoxyacetic acid, which is applied in the field of preparation of linking agent, and can solve the problem of the synthesis method of 4-hydroxymethyl-3-methoxyphenoxyacetic acid, which has not been reported in the literature and other problems, to achieve the effect of easy industrial production, high yield and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
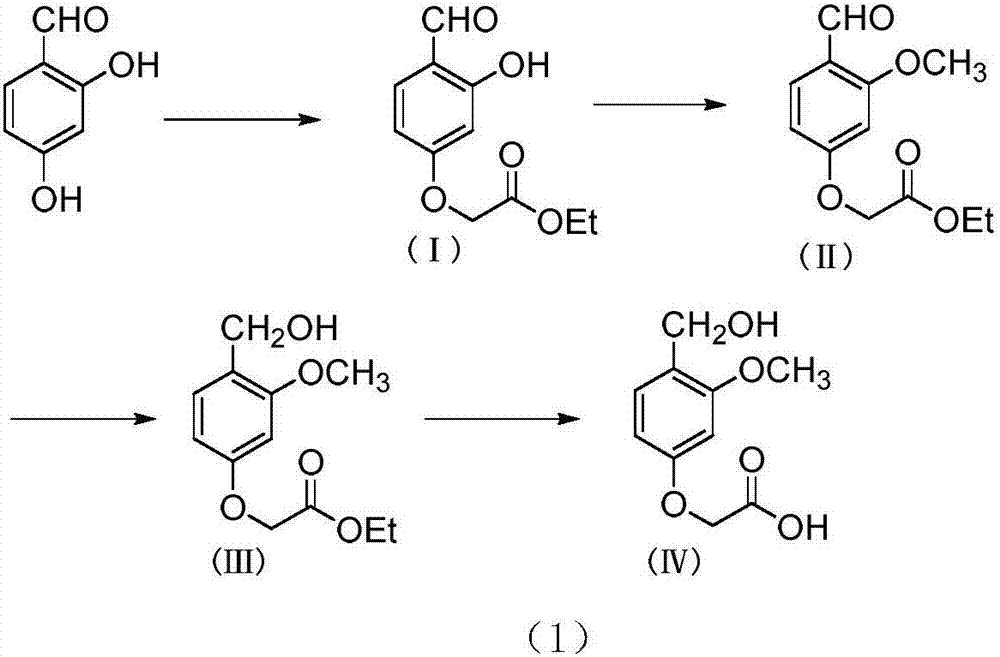
[0047] 1.1), dissolve 5.0g of 2,4-dihydroxybenzaldehyde in 20mL of N,N-dimethylformamide in a one-mouth bottle, add 7.5g of potassium carbonate (Sinopharm Group Shanghai Chemical Reagent Company, chemically pure), and then Add 9.07 g of ethyl bromoacetate (Sinopharm Shanghai Chemical Reagent Company, chemically pure), and react at room temperature. The reaction was carried out for 12 hours, and TLC detected that the reaction of the raw materials was complete. Evaporate N,N-dimethylacetamide to dryness, add ethyl acetate, wash with water, separate the organic phase, dry over anhydrous sodium sulfate, evaporate to dryness, use ethyl acetate to petroleum ether volume ratio of 1:8 for beating and purification . 4.96 g of ethyl 2-(4-formyl-3-hydroxyphenoxy)acetate of formula (I) was obtained with a yield of 69%.
[0048] 1H NMR: (400MHz, CDCl3): δ=1.24(t, J=7.1Hz, 3H), 4.22(q, J=7.2Hz, 2H), 4.60(s, 2H), 6.32(d, J=2.0Hz ,1H),6.52(dd,J=8.7,2.2Hz,1H),7.40(d,J=8.7Hz,1H),9.67(s,1H),1...
Embodiment 2
[0060] 2.1) In a single-necked bottle, dissolve 7.0g of ethyl 2-(4-formyl-3-methoxyphenoxy)acetate in 35mL of acetonitrile, drop it into an ice-water bath, and add 1.88g of 60% hydrogenated Sodium, then add 8.88g iodomethane, room temperature reaction. After 8 hours of reaction, TLC detected that the reaction of the raw materials was complete. The solid was removed by suction filtration, the solvent was evaporated to dryness, ethyl acetate was added, washed with water, the organic phase was separated, dried over anhydrous sodium sulfate, evaporated to dryness, and purified by beating with anhydrous ethanol and petroleum ether at a volume ratio of 3:1. 4.83 g of ethyl 2-(4-formyl-3-methoxyphenoxy)acetate of formula (II) was obtained with a yield of 65%.
[0061] 1H NMR: (400MHz, CDCl3): δ=1.24(t, J=7.1Hz, 3H), 3.83(s, 3H), 4.22(q, J=7.1Hz, 2H), 4.61(s, 2H), 6.41 (dd,J=8.7,1.5Hz,1H),6.47(d,J=1.8Hz,1H),7.73(d,J=8.7Hz,1H),10.23(s,1H).
[0062] MS (EI): m / z=238.
[0063] 2.2) I...
Embodiment 3
[0076] 3.1) Dissolve 4.97g of ethyl 2-(4-formyl-3-methoxyphenoxy)acetate in 25mL of absolute ethanol in a single-necked bottle, drop it into an ice-water bath, add 0.47g of sodium borohydride, React at room temperature. The reaction was carried out for 3 hours, and TLC detected that the reaction of the raw materials was complete. Water was added to the system, extracted with ethyl acetate, washed with saturated sodium bicarbonate solution, dried, evaporated to dryness, and purified by beating with a volume ratio of ethyl acetate to petroleum ether of 1:3. 3.75 g of ethyl 2-(4-hydroxymethyl-3-methoxyphenoxy)acetate of formula (III) was obtained with a yield of 75%.
[0077] 1H NMR: (400MHz, CDCl3): δ=1.23(t, J=7.1Hz, 3H), 2.09(t, J=6.4Hz, 1H), 3.77(s, 3H), 4.21(q, J=7.1Hz ,2H),4.54(d,J=4.2Hz,4H),6.31(dd,J=8.2,2.2Hz,1H),6.50(d,J=2.1Hz,1H),7.09(d,J=8.2Hz ,1H).
[0078] MS (EI): m / z=240.
[0079] 3.2) Dissolve 4.97g of ethyl 2-(4-formyl-3-methoxyphenoxy)acetate in 25mL of abs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com