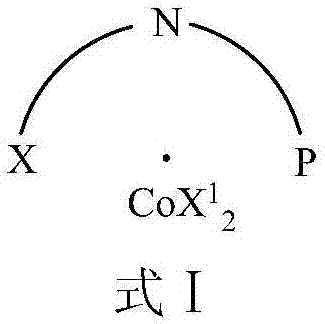
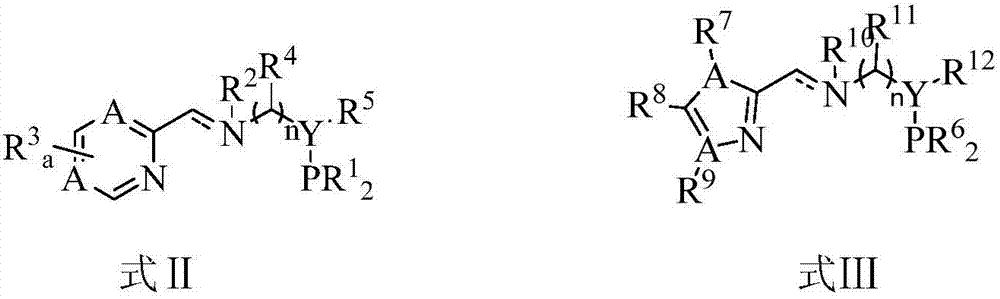
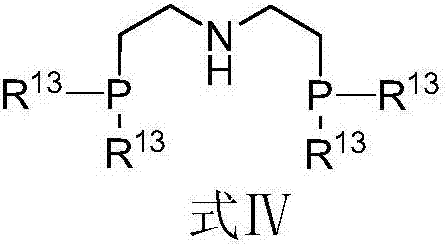
Cobalt complex, preparation method thereof, and application thereof in selective catalysis of transfer hydrogenation reaction of cyano group
A transfer hydrogenation and complex technology, which is applied in catalytic reaction, amino compound preparation, organic compound preparation, etc., achieves high research value and application prospect, simple preparation and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] 2. Preparation of N-Me-Py-NNP ligand
[0075] 1. Synthesis of di-tert-butylphosphine borane complex
[0076]
[0077] Under the protection of argon, add BH 3 • THF solution (24 mL of 1M solution in THF, 24 mmol). After the reaction was stirred at room temperature for 4 h, the solvent was drained to obtain a white solid (2.74 g, 90%). 1 H NMR (400MHz, CDCl 3 )δ3.84( 1 J H-P =345Hz, 3 J H-H =6.5Hz, 1H), 1.25(d, J=13.6Hz, 18H), 0.57(m brw, 3H). 13 C NMR (101MHz, CDCl 3 )δ30.5(d,J C-P =27.5Hz), 28.9. 31P NMR (162MHz, CDCl 3 )δ48.00(q).
[0078] 2. Synthesis of 2-di-tert-butylphosphinoethylamine borane complex
[0079]
[0080] Under argon, add di-tert-butylphosphine borane complex (1.52g, 10mmol) and n-hexane (20ml) to a 100mL Shrek bottle, cool the mixture to -78°C, Add n-butyllithium (2.5M, 8.8mL, 22mmoL) dropwise to the system. After the addition, the reaction was raised to room temperature, and stirring was continued for 1 h to obtain tBu 2 PLi·BH ...
Embodiment 1
[0094] Embodiment 1, synthetic pyridine NNP-CoCl 2 Complex compound (shown in formula 2)
[0095] The reaction equation is as follows:
[0096]
[0097] A solution of degassed THF (5ml) in which pyridine NNP ligand (0.28g, 1.0mmol) was dissolved was added to THF (5ml) of cobalt dichloride (0.115g, 0.90mmol). After the ligand was added, the reaction was suspended The color of the liquid changed from blue to pink, stirred at room temperature (25°C) for 12 hours, then concentrated the solution to 2ml, added 15ml of degassed n-hexane, stirred for 1 hour, filtered with argon, and dried the obtained filter cake to obtain a pink solid (0.339 g, 83%).
[0098] Elemental analysis: theoretical value (%): C, 46.84; H, 7.13; N, 6.83; measured: C, 46.82; H, 7.34; N, 6.62.
[0099] NMR 1 H NMR (400MHz, CD 2 Cl 2 )δ=50.72(br),44.82(br),41.24(br),34.48(br),3.27(br),2.57(br),2.32(br),1.37(br),-2.88(br).
[0100] Magnetic distance: 4.3μ B.
[0101] Infrared (KBr): 3430, 2951, 1607, ...
Embodiment 2
[0103] Embodiment 2, synthesis Py-imine-NNP-CoCl 2 Complex compound (shown in formula 3)
[0104] The reaction equation is as follows:
[0105]
[0106] A solution of degassed THF (5 mL) in which the Py-imine-NNP ligand (0.278 g, 1.0 mmol) was dissolved was added in THF (5 mL) of cobalt dichloride (0.115 g, 0.90 mmoL). After the ligand was added, The reaction was stirred at room temperature (25° C.) for 8 h, then the solution was concentrated to 2 mL, 15 mL of degassed n-hexane was added, stirred for 1 hour, filtered with argon, and the obtained filter cake was dried to obtain a brown solid (0.330 g, 91%).
[0107] Elemental analysis: theoretical value (%): C, 47.08; H, 6.67; N, 6.86; measured: C, 46.60; H, 6.37; N, 7.12.
[0108] NMR: 1 H NMR (400MHz, CD 2 Cl 2 )δ=60.94(br),51.19(br),48.81(br),3.48(br),1.94(br),0.90(br),-0.59(br).
[0109] Magnetic distance: 4.2μ B.
[0110] Infrared: (KBr): 3433, 3060, 2944, 2867, 1653, 1600, 1567, 1470, 1445, 1394, 1372, 1299, 11...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap