A fluorescent probe for detecting silver ions and its preparation method and application
A technology of fluorescent probes and silver ions, which is applied in the field of fluorescent probes for detecting silver ions, can solve problems such as fewer silver ion fluorescent probes, low sensitivity, and environmental hazards, and achieve easy scale production, high sensitivity, and low environmental impact Harmful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-1
[0043] The preparation method of embodiment I-1 fluorescent probe I of the present invention, its steps comprise:
[0044] (1) Synthesis of indazole
[0045] Add 21.4g (200mmol) of o-methylaniline, 50mL (600mmol) of concentrated hydrochloric acid and 100mL of water into the reaction flask, stir at 0°C, add dropwise 13.8g (200mmol) of an aqueous solution of sodium nitrite, after the addition is complete Keep stirring at 0°C for 30 minutes; add sodium fluoroborate solution (24.2g (200mmol) dissolved in 100mL water), keep at 0°C for 2 hours, a large amount of white diazonium salt precipitates in the reaction bottle; pump the reaction solution at low temperature The diazonium salt was obtained by filtration, washed with 300 mL of ice-cold ethanol, and dried to obtain 28.5 g of the diazonium salt, with a yield of 46.1%.
[0046] Dissolve the diazonium salt in 50mL chloroform and add it to the reaction flask, stir at 0°C, add 19.8g (200mmol) potassium acetate solid in batches, retu...
Embodiment I-2
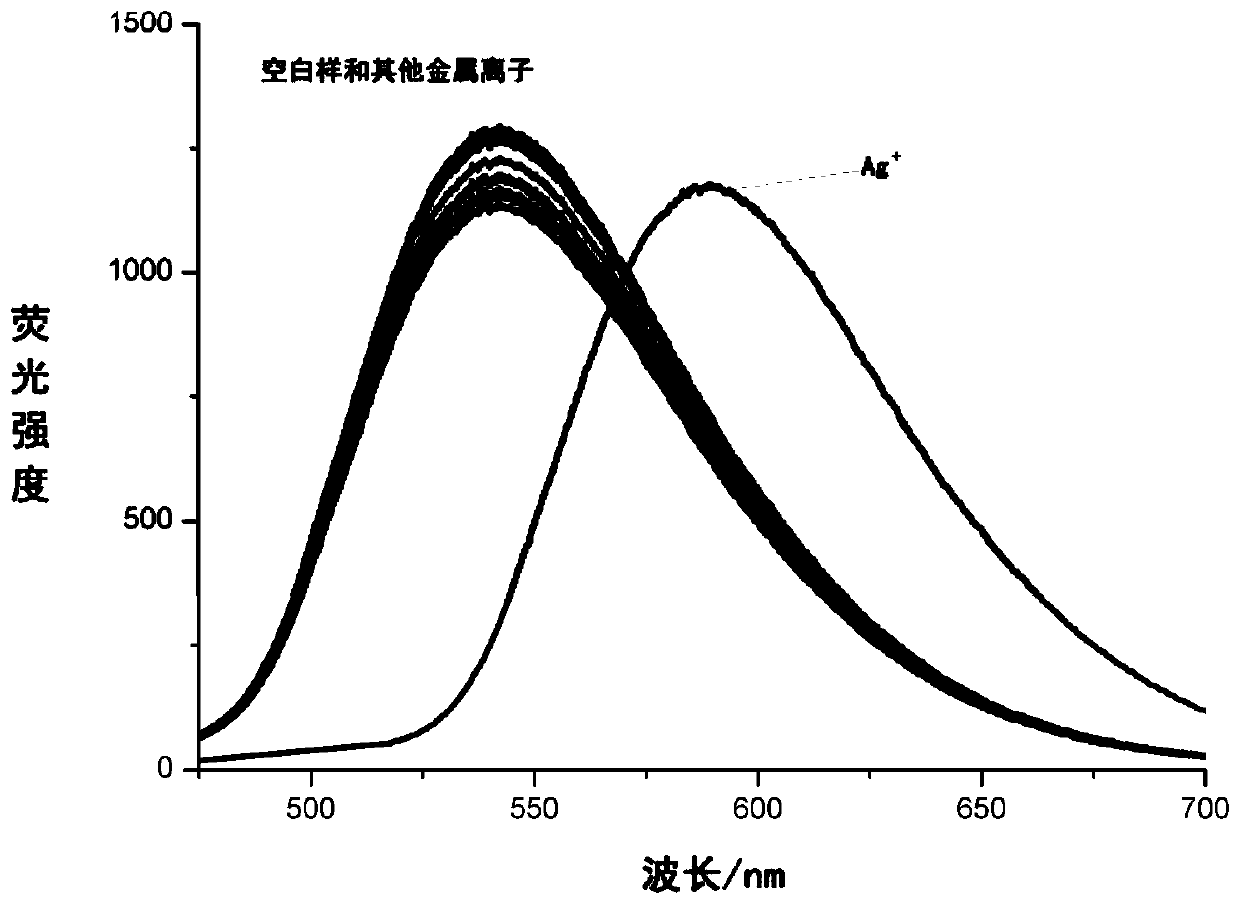
[0057] Spectral properties of embodiment 1-2 silver ion fluorescent probe 1 and various metal ions
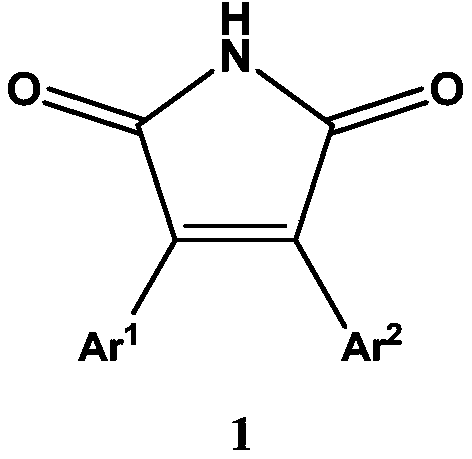
[0058] The structure of fluorescent probe I is characterized in that it has a structural formula as shown in formula 9:
[0059]
[0060] Fluorescent probe I was prepared in tetrahydrofuran to 1.0 × 10 -5 mol / L mother liquor, packed in ten 5mL glass bottles. Ten metals (Ag(I), Li(I), Cu(II), Zn(II), Hg(II), Ni(II), Al(III), Fe(III), Cr(III)) The salt is dissolved in water, respectively dubbed 5.0×10 -3 mol / L metal salt solution. Add 10 μL of these 10 metal salt solutions to these 10 glass bottles, so that the final concentration of metal ions in each bottle is 1.0×10 -4 mol / L test sample. After ultrasonic vibration for 30 minutes, take the test sample and test its fluorescence emission spectrum under the excitation light wavelength of 450nm, the obtained results are as follows: figure 1 shown.
[0061] This experiment shows that the fluorescent probe 1 has high select...
Embodiment I
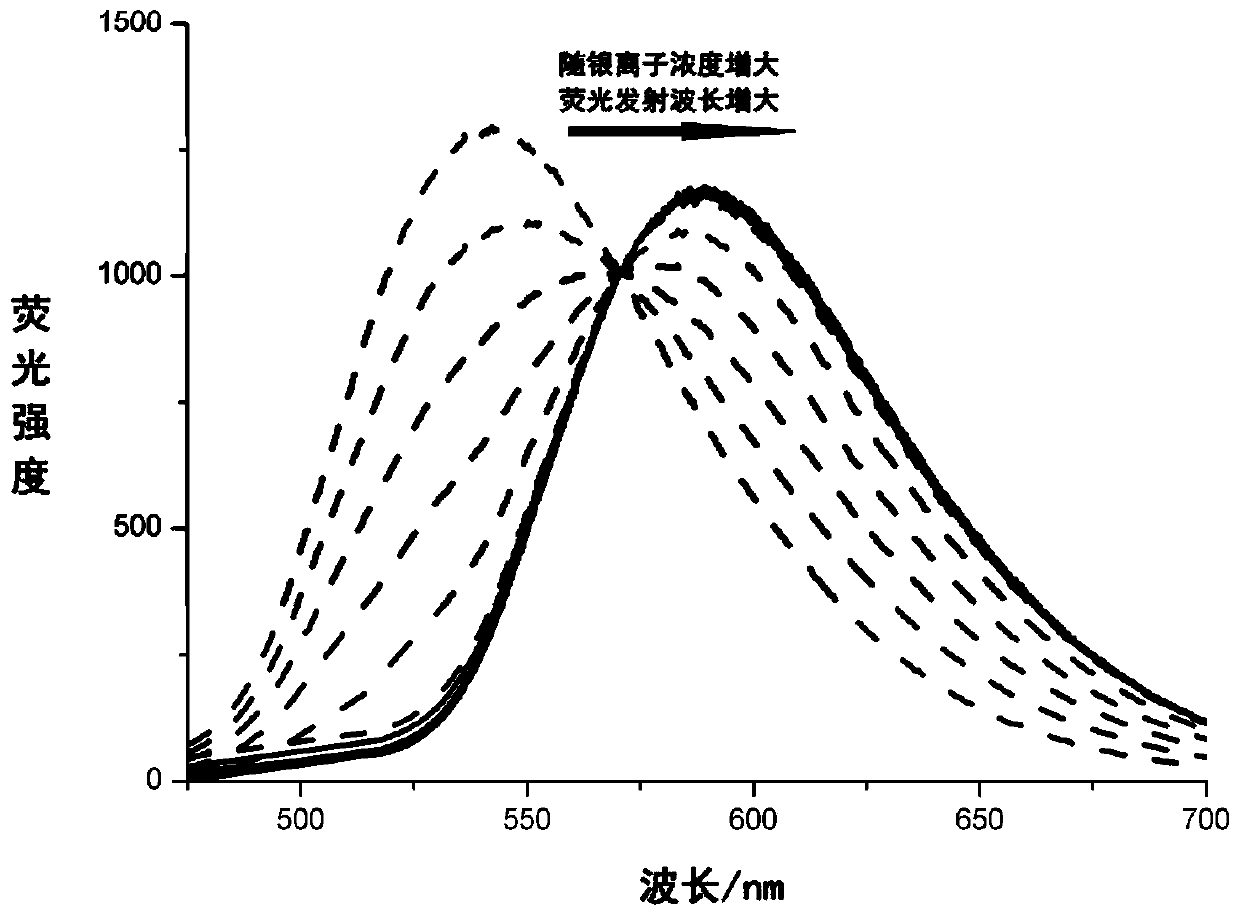
[0062] Embodiment I-3 Spectral properties of the reaction product of fluorescent probe I and silver ions.
[0063] The mother solution of fluorescent probe I in Example I-1 was filled into 10 5mL glass bottles respectively. Mix silver nitrate into 0.5×10 -3 mol / L, 1.0×10 -3 mol / L, 1.5×10 -3 mol / L, 2.0×10 -3 mol / L, 2.5×10 -3 mol / L, 3.0×10 - 3 mol / L, 3.5×10 -3 mol / L, 1.5×10 -3 mol / L, 4.0×10 -3 mol / L, 4.5×10 -3 mol / L and 5.0×10 -3 mol / L solution. Add 10 μL of these 10 concentrations of silver nitrate solutions to these 10 glass bottles, and these 10 bottles contain test samples with a molar ratio (metal ion: fluorescent probe) of 1:10 to 1:1. After ultrasonic vibration for 30 minutes, take the solution in the bottle and test its fluorescence emission spectrum under the excitation light wavelength of 450nm, the obtained results are as follows: figure 2 shown.
[0064] The experimental results showed that the fluorescence emission peak of fluorescent probe 1 reacted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com