Manganese dioxide modified biochar composite material as well as a preparation method and an application thereof
A technology of manganese dioxide and composite materials, which is applied in the field of materials and the environment, can solve the problems of insufficient utilization of manganese elements, waste of raw materials, and difficulty in controlling the load of manganese oxides, etc., to achieve effective control, obvious adsorption effect, and increased stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
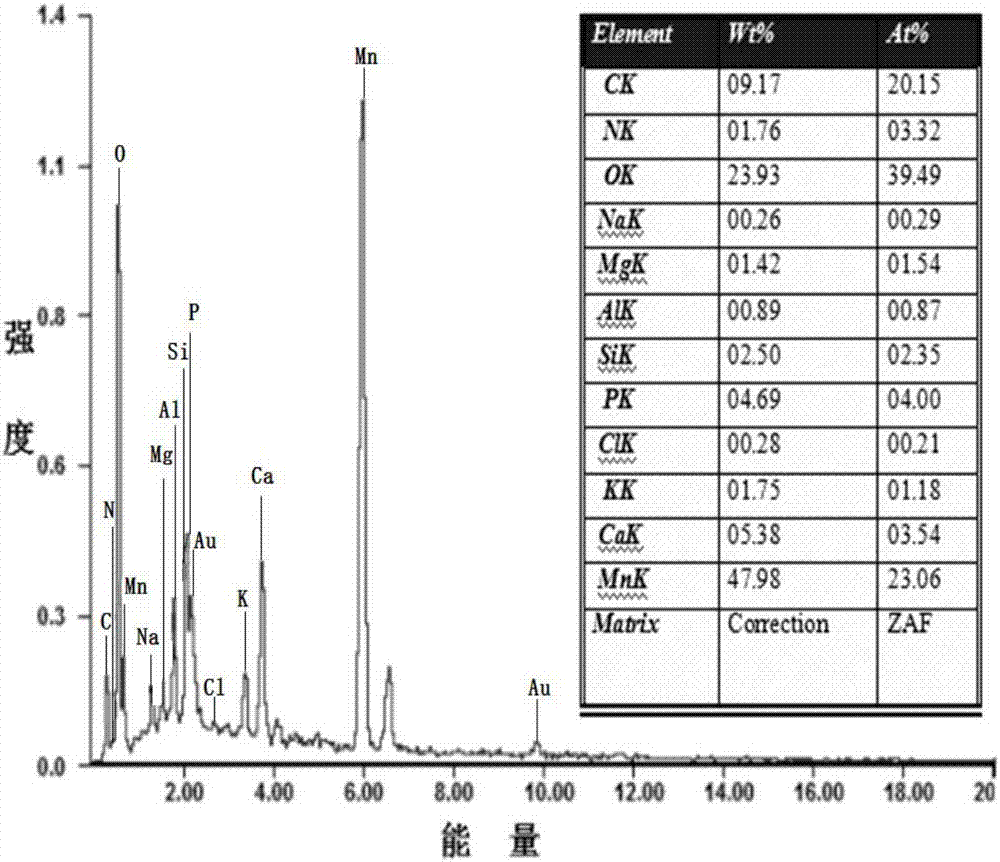
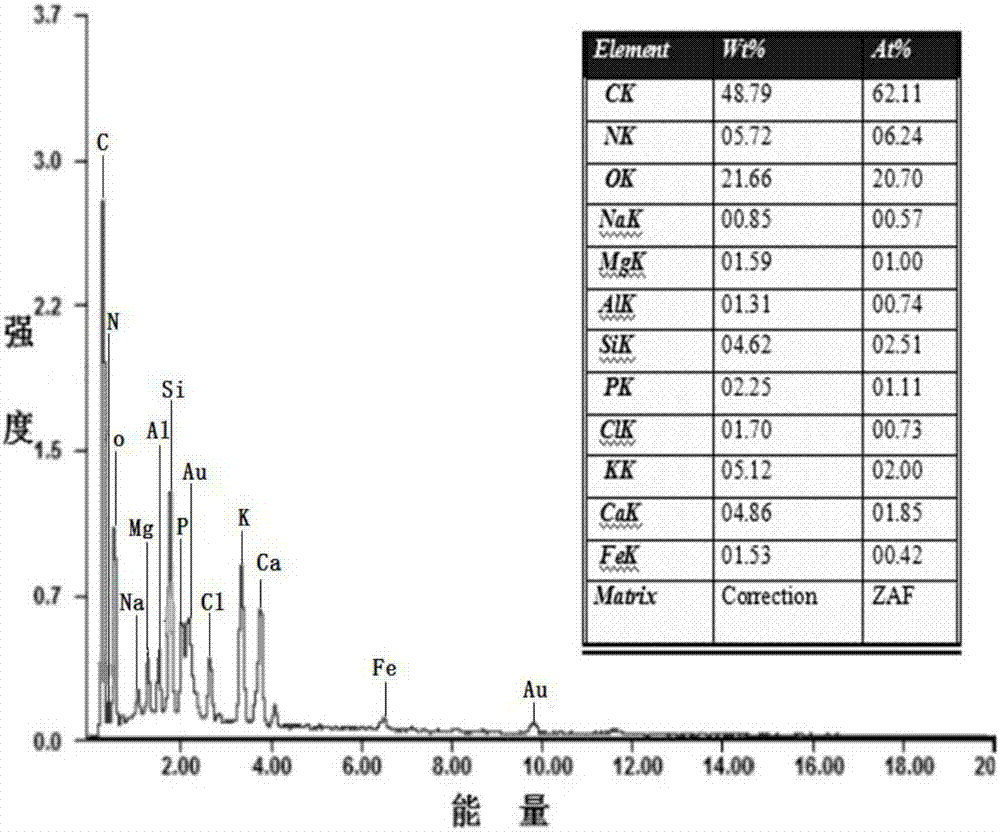
[0055] A biochar composite material modified by manganese dioxide of the present invention comprises manganese dioxide and biochar; the biochar composite material modified by manganese dioxide is obtained through the normalization of manganese elements in potassium permanganate and manganese acetate tetrahydrate The reaction is prepared by loading the generated manganese dioxide on biochar.
[0056] A preparation method of the manganese dioxide modified biochar composite material of the above-mentioned present embodiment, comprising the following steps:
[0057] S1. Mix pig manure and camphor wood chips in a mass ratio of 4:1, and perform aerobic composting for 60 days to obtain the mixture, and wash the mixture several times with deionized water, dry it at 80°C, grind it, and pass it through a 100-mesh sieve , and store in a sealed bag for later use.
[0058] S2. Put the mixture passed through a 100-mesh sieve in step S1 into a quartz boat, then put the quartz boat in a tube...
Embodiment 2
[0070] An application of the manganese dioxide-modified biochar composite material of the present invention in the treatment of heavy metal wastewater, wherein the manganese dioxide-modified biochar composite material is derived from Example 1, and the heavy metal wastewater is lead-containing heavy metal wastewater or cadmium-containing heavy metal wastewater .
[0071] Process lead-containing heavy metal wastewater, comprising the following steps: take by weighing 13 parts of manganese dioxide-modified biochar composite materials in Example 1 in an Erlenmeyer flask, adding an ionic strength of 0.01mol / L , Lead-containing heavy metal wastewater with a pH value of 5.0±0.2, wherein the initial concentrations of lead in the lead-containing heavy metal wastewater are 0.5mg / L, 1.0mg / L, 5.0mg / L, 10.0mg / L, 20.0mg / L, 40.0 mg / L, 60.0mg / L, 80.0mg / L, 100.0mg / L, 120.0mg / L, 150.0mg / L, 180.0mg / L and 210.0mg / L. After mixing, the adsorption reaction was shaken for 12 hours under the conditi...
Embodiment 3
[0083] An application of the manganese dioxide-modified biochar composite material of the present invention in the treatment of heavy metal wastewater, the manganese dioxide-modified biochar composite material is derived from Example 1, and the heavy metal wastewater is lead-containing heavy metal wastewater or cadmium-containing heavy metal wastewater .
[0084] The treatment of lead-containing heavy metal wastewater includes the following steps: weighing 39 parts of the manganese dioxide-modified biochar composite material in Example 1 in an amount of 0.40 g / L in 39 Erlenmeyer flasks, and dividing them into three groups. Add lead-containing heavy metal wastewater with an ionic strength of 0.01mol / L and a pH value of 5.0±0.2 into each group of Erlenmeyer flasks. The initial concentrations of lead in the lead-containing heavy metal wastewater are 0.5mg / L, 1.0mg / L, and 5.0mg respectively. / L, 10.0mg / L, 20.0mg / L, 40.0mg / L, 60.0mg / L, 80.0mg / L, 100.0mg / L, 120.0mg / L, 150.0mg / L, 180...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com