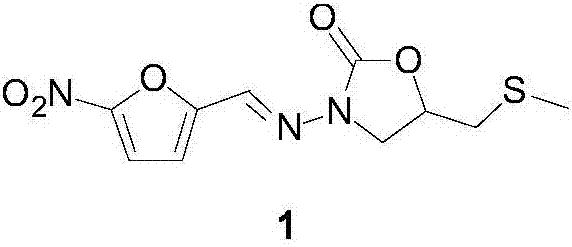
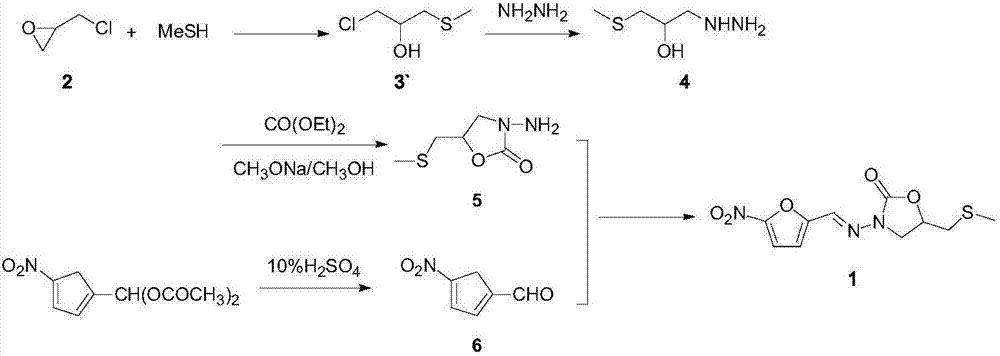
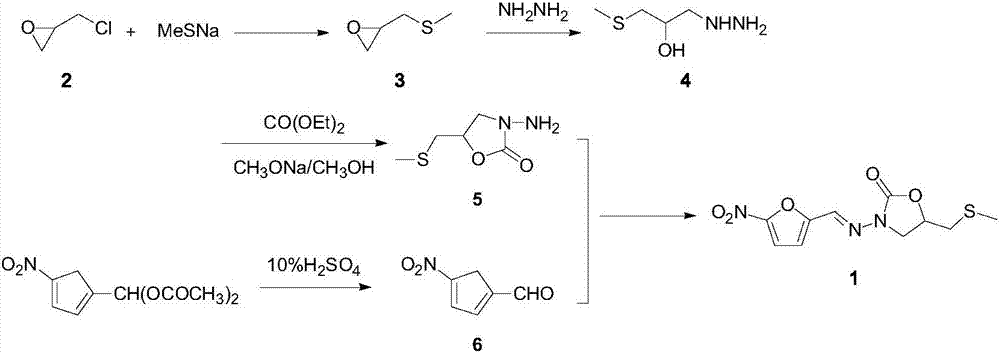
Preparation process of anti-infective drug nifuratel
A technology for preparing nifuratel, which is applied in the field of drug synthesis, can solve the problems of high risk of sodium hydride, many side reactions, and difficult operation, and achieve cheap and easy-to-obtain raw material reagents, improve purity and yield, and reduce The effect of operational difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of glycidyl methyl sulfide:
[0053] In a 1000ml reaction bottle, add 78g of sodium sulfide and 234ml of water, start stirring, dropwise add 284g of methyl iodide, control the rate of addition so that the temperature of the reaction system does not exceed 25°C, keep warm for 1.0h after the dropwise addition, and then add chlorine dropwise Substitute cyclopropane 276g, keep warm for 2.0h after the completion of the dropwise addition, separate the liquid after the completion of the heat preservation, the organic phase weighs 178.80g, the gas phase detection purity is 96.35%, that is, glycidyl methyl sulfide, and the yield is 85.96%.
Embodiment 2
[0055] Preparation of glycidyl methyl sulfide:
[0056] In a 1000ml reaction bottle, add 124.80g of sodium sulfide and 234ml of water, start stirring, dropwise add 284g of methyl iodide, control the rate of addition, so that the temperature of the reaction system does not exceed 25°C, keep warm for 1.0h after the addition is completed, and dropwise add Chlorocyclopropane 184g, heat preservation 2.0h after completion of the dropwise addition, liquid separation after completion of heat preservation, the organic phase weighed 173.66g, gas phase detection purity 97.2%, namely glycidyl methyl sulfide, yield 84.93%.
Embodiment 3
[0058] Preparation of 3-methylthio-2-hydroxy-propylhydrazine:
[0059] In a 1000ml reaction flask, add 226.25g of hydrazine hydrate, heat to 90°C, add 104.17g of glycidyl methyl sulfide prepared in Example 1 dropwise under stirring, control the rate of addition so that the temperature of the reaction system is controlled at 90°C, add After heat preservation for 1.0 h, excess hydrazine hydrate was concentrated under reduced pressure to obtain 120.10 g of a viscous colorless liquid with a gas phase detection purity of 96.8%, namely 3-methylthio-2-hydroxy-propylhydrazine, yield 88.30% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com