Modified polyethylene terephthalate and preparation method thereof
A technology of ethylene glycol phthalate and terephthalic acid, which is applied in the field of preparation of modified polyethylene terephthalate, can solve problems such as molecular weight reduction, achieve no molecular weight reduction, increase crystallization temperature, The effect of increasing crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
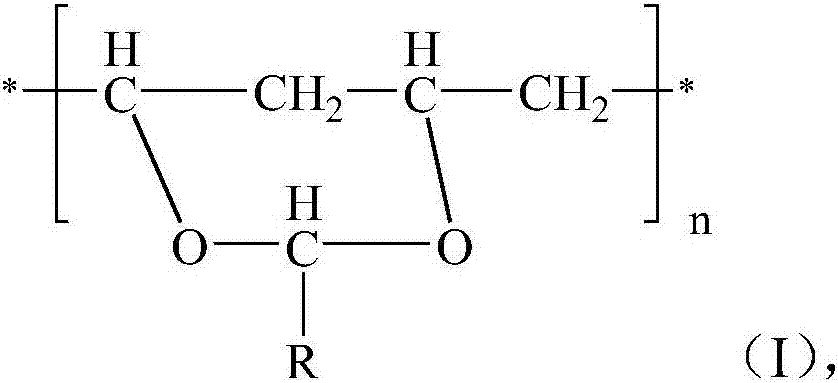
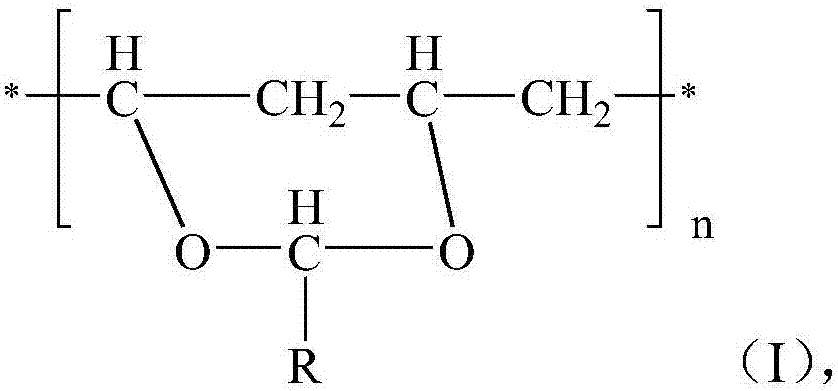
[0018] The invention provides a method for preparing modified polyethylene terephthalate, comprising: (1) reacting ethylene glycol, terephthalic acid and a crystallization accelerator in the presence of a catalyst to obtain a reaction product; (2) Remove excess unreacted ethylene glycol from the reaction product, and then carry out polycondensation under polycondensation reaction conditions to obtain modified polyethylene terephthalate; wherein, the crystallization accelerator is selected from the formula The polyvinyl acetal shown in (I),
[0019]
[0020] Among them, R is H or C 1 ~C 10 The alkyl group, n=200~1000.
[0021] According to the present invention, preferably R in formula (I) is H or n-propyl, and preferably, the polyvinyl acetal is polyvinyl formal or polyvinyl butyral.
[0022] According to the present invention, preferably, the number average molecular weight of the polyvinyl acetal is 30000-60000.
[0023] In the present invention, the polyvinyl acetal ...
Embodiment 1
[0051] This example illustrates the preparation method of the modified polyethylene terephthalate of the present invention.
[0052] Feeding see Table 1.
[0053] (1) Reaction: 500g of terephthalic acid (PTA), 250g of ethylene glycol (EG), 0.43g of tetrabutyl titanate, 0.54g of neodymium acetylacetonate, 0.16g of tributyl phosphate and 0.5 g of polyvinyl formal (PVF) was added to a 2.5L polymerization kettle for reaction. The reaction temperature is 240° C., the reaction pressure is 0.2 MPa, and the reaction time is 150 min. The water produced by the reaction is discharged through the rectification device. After the reaction finishes, the polymerization kettle pressure is reduced to normal pressure;
[0054] (2) Polycondensation reaction: Vacuumize the polymerization kettle to 600Pa to discharge unreacted ethylene glycol for 80 minutes; continue to reduce the pressure to 200Pa, and raise the temperature of the polymerization kettle to 270°C for polycondensation reaction at ...
Embodiment 2
[0057] This example illustrates the preparation method of the modified polyethylene terephthalate of the present invention.
[0058] Feeding see Table 1.
[0059] (1) Reaction: 500g of terephthalic acid (PTA), 187g of ethylene glycol (EG), 0.62g of lanthanum chloride, 0.42g of tetrabutyl titanate, 0.12g of trimethyl phosphate and 1.0 g of polyvinyl formal (PVF) was added to a 2.5L polymerization kettle for reaction. The reaction temperature is 220° C., the reaction pressure is 0.4 MPa, and the reaction time is 30 minutes. The water produced by the reaction is discharged through the rectification device. After the reaction finishes, the polymerization kettle pressure is reduced to normal pressure;
[0060] (2) Polycondensation reaction: Vacuumize the polymerization kettle to 500Pa to discharge unreacted ethylene glycol for 60min; continue to reduce the pressure to 100Pa, and raise the temperature of the polymerization kettle to 290°C for polycondensation reaction at the same...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com