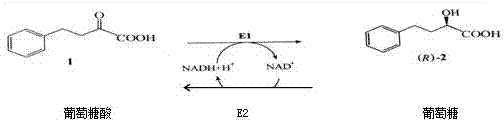
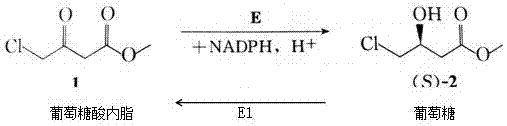
Keto group reduction catalytic system construction and cyclic operation method
A catalytic system and cycle operation technology, applied in the direction of fermentation, etc., can solve the problems of no effective recovery of coenzyme I, complicated and difficult recycling, etc., and achieve the effects of reducing production costs, solving technical bottlenecks, and simple and effective recovery means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Escherichia coli ketoreductase engineering strain ¸Escherichia coli glucose dehydrogenase engineering strain was expanded and cultured separately, and 100ml of seed solution was prepared for each, and inoculated in 15L sterilized TB medium respectively, and the pH was controlled at 7.0, and the culture temperature was 37°C, the maximum speed of the fermenter is 500 rpm; after the dissolved oxygen rebounds, add 60% glycerin solution at a rate of 55ml / h; about 8 hours, the OD600 reaches 20, cool down to 30°C, and press into the sterile-filtered IPTG solution (content 1.2g) for induction, after 6 hours of induction, put it into the tank. The enzyme activity of the fermentation broth was detected, which were 16.5ug / ml and 22.3ug / ml respectively.
[0022] (2) Collect the cells by centrifugation at 8000 rpm, and obtain 1246g and 1356g cells respectively; take 600g of ketoreductase cells and 400g of glucose dehydrogenase cells respectively, and weigh them with 4L of 50mM p...
Embodiment 2
[0033] (1) Escherichia coli glucose dehydrogenase engineering strain was expanded and cultivated, and 100 ml of seed solution was prepared, and 1450 ml of glucose dehydrogenase enzyme solution was prepared according to the method provided in Example 1.
[0034] (2) Expand the cultivation of Pichia ketoreductase engineering strains, prepare 300ml of seed liquid, inoculate in 15L of sterilized medium, medium ratio: phosphoric acid 26.7g / L, calcium sulfate 0.93g / L, Potassium sulfate 18.2g / L, magnesium sulfate 14.9g / L, potassium hydroxide 4.31g / L, glycerin 40.0g / L, defoamer 0.4g / L, trace elements 5ml / L, adjust pH to 5.6 with ammonia water; Control pH 5.0, culture temperature 30°C, maximum speed 500 rpm; start to add 50% glycerin solution after dissolved oxygen rebounds, and the rate should be controlled at about 30% dissolved oxygen; about 6 hours, the wet weight of cells reaches 160g / L, stop supplementing glycerin; after the dissolved oxygen rebounds, add methanol, the total dos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com