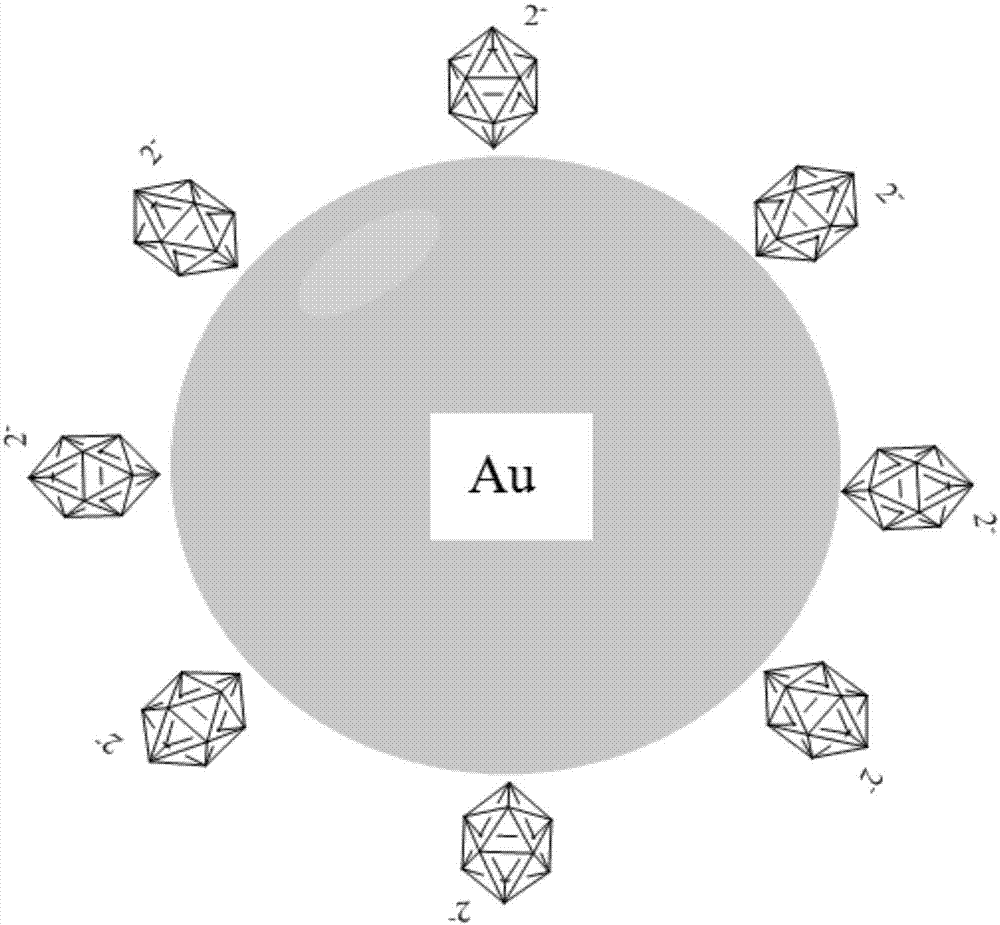
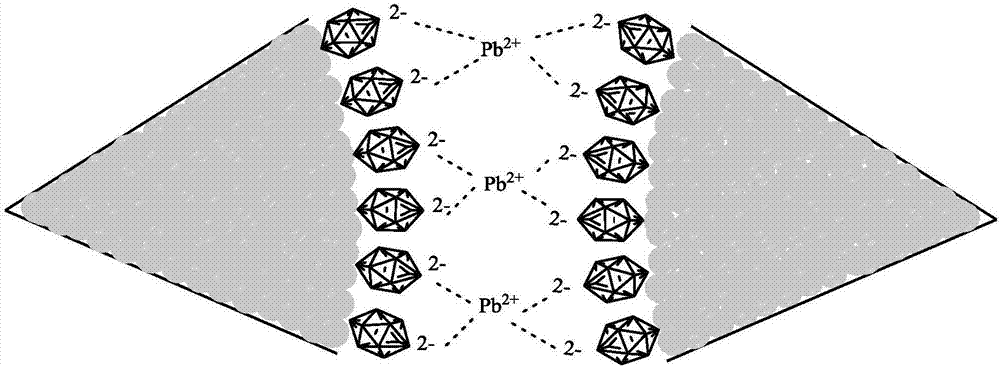
Nanometer gold colorimetry method for rapidly detecting lead ions
A nano-gold colorimetry, lead ion technology, applied in the field of analytical chemistry, can solve problems such as difficult operation and complicated process, and achieve the effect of high application value, mild reaction conditions and strong resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) AuNPs-B 12 h 12 2- Solution preparation
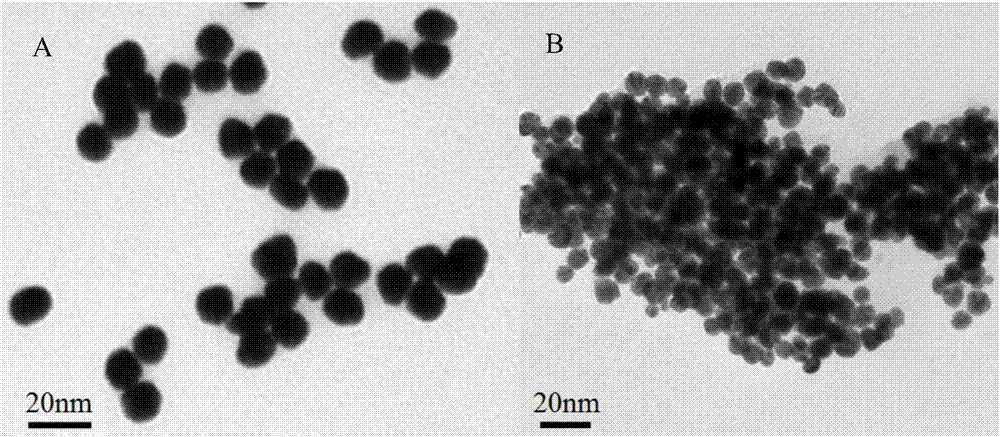
[0032] The present invention uses Na 2 B 12 h 12 Gold nanoparticles (AuNPs) were prepared by reduction of chloroauric acid (the transmission electron microscope picture is shown in image 3 (A) shown). First, soak all the glass instruments and magnets with aqua regia, add 1g HAuCl 4 4H 2 O was dissolved in 100mL distilled water to obtain a chloroauric acid solution with a concentration of 1wt%; accurately weighed 0.9895g Na 2 B 12 h 12 Dissolve in 100 mL of distilled water to obtain a concentration of 8×10 -5 mol / L Na 2 B 12 h 12 solution.
[0033] Get 1mL 1wt% chloroauric acid solution, add in the 250mL round bottom flask that fills 100mL deionized water, stir at room temperature; The molar ratio of salt to chloroauric acid is 0.1 ~ 1:1), quickly added to the above 250mL round bottom flask, the mixed solution changed from colorless to purple after 1 minute, and continued to stir for 30 minutes to obtain AuNP...
Embodiment 2
[0043] Compared with Embodiment 1, the only difference is that in this embodiment:
[0044] (1) Using cesium dodecahydrododecaborate (Cs 2 B 12 h 12 ) to prepare burgundy AuNPs-B by reducing chloroauric acid 12 h 12 2- Solution, wherein, the concentration of nano gold is 0.8nmol / L, [B 12 h 12 ] 2- The concentration is 4×10 -5 mol / L, the particle size of nano gold is 14nm;
[0045] (2) Add an appropriate amount of NaOH solution to the solution obtained in step (1), and adjust the pH of the solution to 10;
[0046] (3) adding an appropriate amount of NaCl solution to the solution obtained in step (2), controlling the concentration of NaCl to be 80mmol / L;
[0047] (4) in the solution that step (3) obtains, add the Pb to be measured 2+ solution, mixed evenly, and measure the ultraviolet-visible absorption spectrum of the solution after 20 minutes to obtain the ultraviolet-visible absorption spectrum corresponding to different concentrations of lead ions. Then use the a...
Embodiment 3
[0049] Compared with Embodiment 1, the only difference is that in this embodiment:
[0050] (1) Potassium dodecahydrododecaborate (K 2 B 12 h 12 ) to prepare burgundy AuNPs-B by reducing chloroauric acid 12 h 12 2- Solution, wherein, the concentration of nano gold is 2.2nmol / L, [B 12 h 12 ] 2- The concentration is 6×10 -5 mol / L, the particle size of nano gold is 10nm;
[0051] (2) Add an appropriate amount of NaOH solution to the solution obtained in step (1), and adjust the pH of the solution to 12;
[0052] (3) adding an appropriate amount of NaCl solution to the solution obtained in step (2), controlling the concentration of NaCl to be 50mmol / L;
[0053] (4) to step (3) obtained - Pb to be tested was added to the solution 2+ solution, mixed evenly, and measure the ultraviolet-visible absorption spectrum of the solution after 15 minutes to obtain the ultraviolet-visible absorption spectrum corresponding to different concentrations of lead ions. Then use the abso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com