Method for preparing human fibrinogen
A human fibrinogen and protein technology, which is applied in the field of human fibrinogen preparation, can solve the problems of plasma waste, backward product technology, low purity, etc., and achieves the effects of good removal of impurities, improved production efficiency, and uniform precipitation and crossover.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
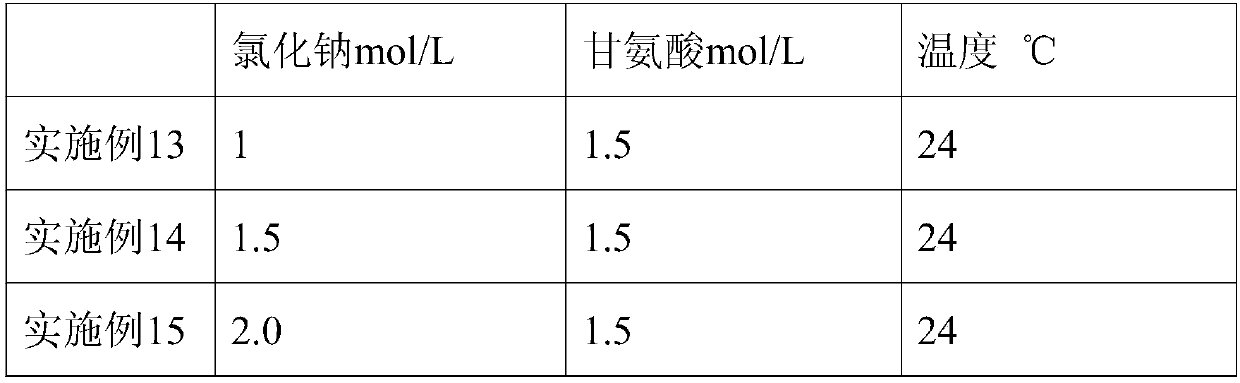
[0032] A preparation method of human fibrinogen, comprising the following preparation steps:
[0033] 1) Plasma melting: the plasma is completely melted at 0°C;
[0034] 2) Ethanol precipitation: adjust the pH of the reacted plasma to 7.0 with acetic acid buffer, adjust the protein concentration of the reaction solution to 5.2%, cool down to -1°C, add 95% ethanol until the final ethanol concentration of the reaction solution is 8%, and adjust the temperature to -1°C, continue to stir for more than 30 minutes after adding ethanol, and collect F by centrifugation I precipitation;
[0035] 3) First extraction: F I Dissolve the precipitate with dissolving solution 1, filter through the filter element, and obtain a clarified filtrate;
[0036] 4) Chromatography: Adsorb the clarified filtrate obtained in step 3) with Toyopeal DEAE-650M gel, the sample loading flow rate is 1.3cm / min, pass through the ultraviolet detector, and after the protein peak is detected, take the flow-throu...
Embodiment 2
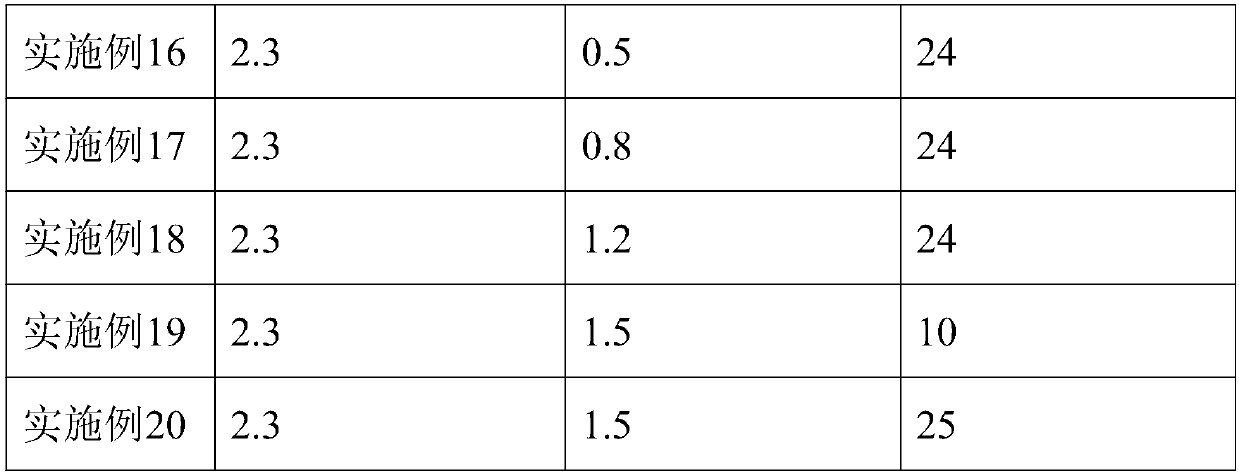
[0051] A preparation method of human fibrinogen, comprising the following preparation steps:
[0052] 1) Plasma melting: the plasma is completely melted at 3°C;
[0053] 2) Ethanol precipitation: adjust the pH of the reacted plasma to 7.3 with acetic acid buffer, adjust the protein concentration of the reaction solution to 5.3%, cool down to 0°C, add 95% ethanol until the final ethanol concentration of the reaction solution is 8%, and adjust the temperature to - 2°C, continue to stir for more than 30 minutes after adding ethanol, and collect F by centrifugation I precipitation;
[0054] 3) First extraction: F I Dissolve the precipitate with solution 1, filter through the filter element, and obtain a clarified filtrate;
[0055] 4) Chromatography: Adsorb the clarified filtrate obtained in step 3) with Toyopeal DEAE-650M gel, the sample loading flow rate is 1.4cm / min, pass through the ultraviolet detector, and after the protein peak is detected, take the flow-through protein ...
Embodiment 3
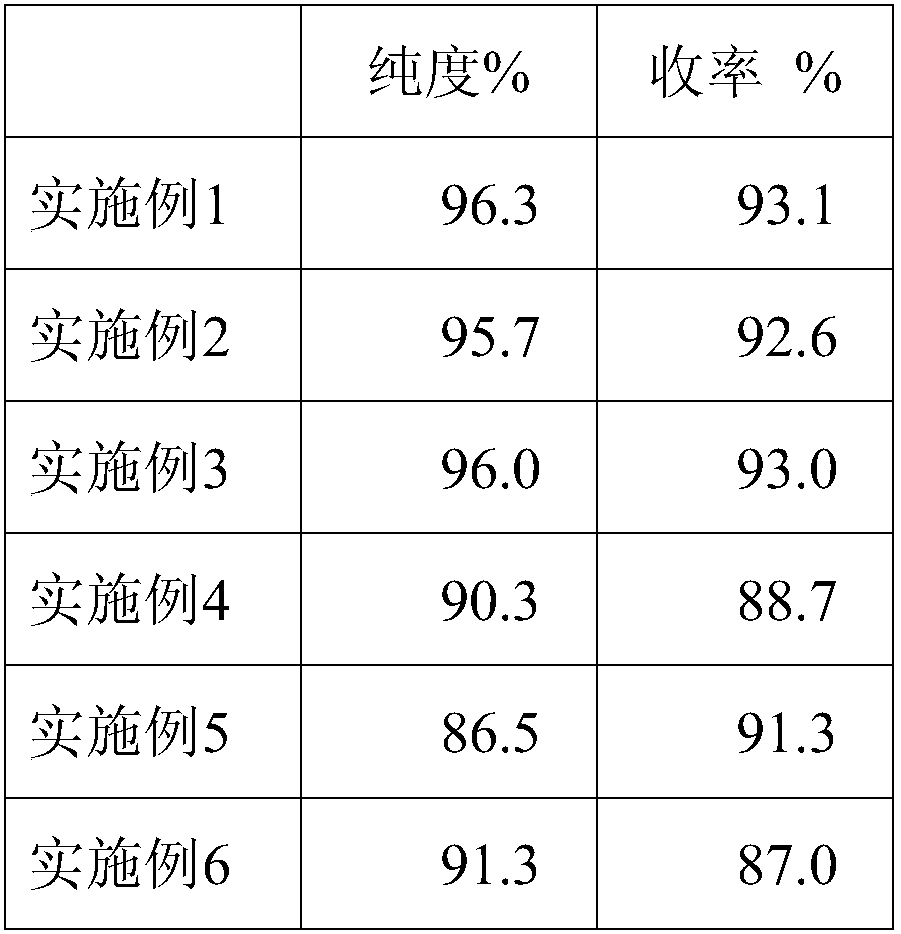
[0070] A preparation method of human fibrinogen, comprising the following preparation steps:
[0071] 1) Plasma melting: the plasma is completely melted at 0-6°C;
[0072] 2) Ethanol precipitation: adjust the pH of the reacted plasma to 7.5 with acetic acid buffer, adjust the protein concentration of the reaction solution to 5.5%, cool down to 1°C, add 95% ethanol until the final ethanol concentration of the reaction solution is 8%, and adjust the temperature to - 3°C, continue to stir for more than 30 minutes after adding ethanol, and collect F by centrifugation I precipitation;
[0073] 3) First extraction: F I Dissolve the precipitate with dissolving solution 1, and filter through the filter element to obtain a clarified filtrate;
[0074] 4) Chromatography: Adsorb the clarified filtrate obtained in step 3) with Toyopeal DEAE-650M gel, the sample loading flow rate is 1.5cm / min, pass through the ultraviolet detector, and after the protein peak is detected, take the flow-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com