Formation of cyclosporin a/cyclodextrin nanoparticles
A technology of cyclosporine and cyclodextrin, which can be used in cyclopeptide components, medical preparations of inactive components, sensory diseases, etc., can solve the problems of itchy conjunctiva, low drug bioavailability, blurred vision, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
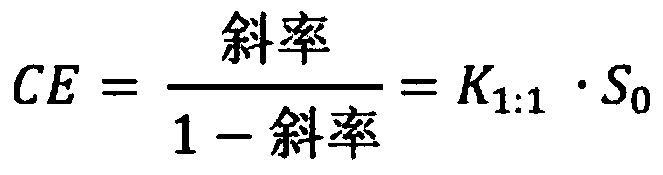
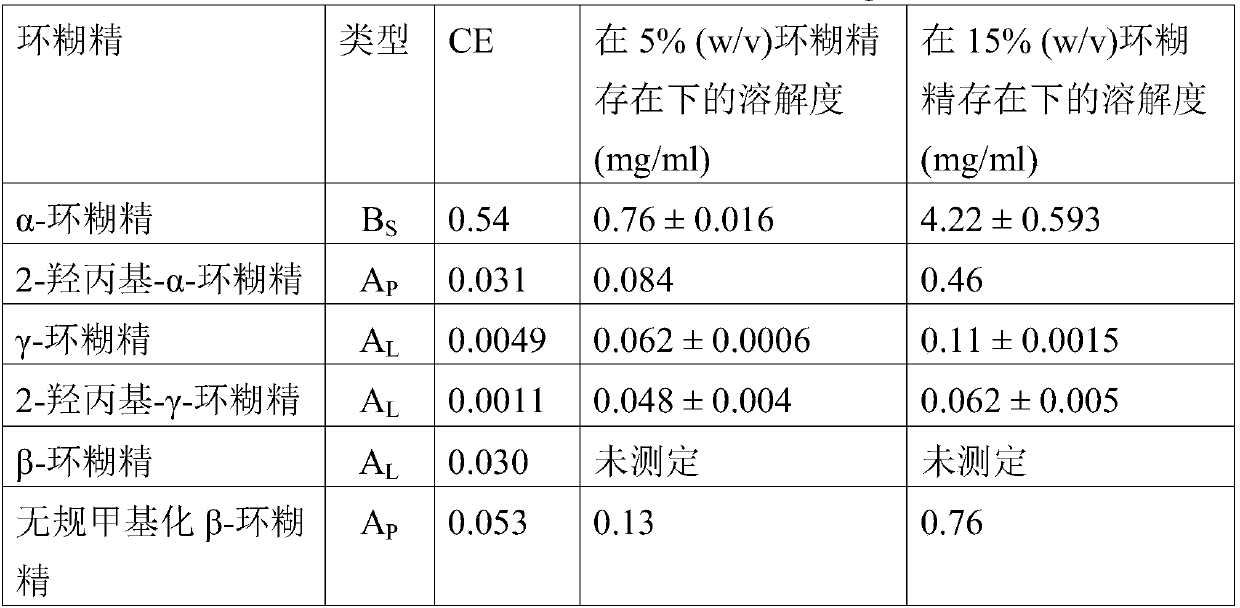
[0083] The effect of cyclodextrin on the solubility of cyclosporin A in water was studied. Excess drug was added to aqueous solutions containing up to 20% (w / v) cyclodextrin. The solution was sonicated in a sealed glass vial at 40-50°C for 45-60 minutes, then allowed to cool to room temperature (22-23°C). A small amount of solid drug was then added to each vial, which was resealed and allowed to equilibrate under constant stirring and protected from light at room temperature for 7 days. When the solution had reached equilibrium, it was filtered through a 0.45 μm membrane filter and analyzed by high pressure liquid chromatography. The apparent complexation constant (K 1:1 ) (Higuchi, T., Connors, K.A., 1965. Phase solubility techniques. Advanced Analytical Chemistry of Instrumentation 4, 117-212). Complexation efficiency (CE) is determined from the slope of a phase solubility diagram (a plot of the total solubility of a drug against the total CD concentration in mol / l), wher...
Embodiment 2
[0091] Determination of the cyclosporin A moiety present in cyclosporin A / cyclodextrin aggregates in aqueous eye drop media. Prepare 0.05% (w / v) cyclosporine by dissolving benzalkonium chloride (20 mg) and edetate disodium dehydrate (100 mg) in 70 ml of a 1.4% (w / v) aqueous solution of polyvinyl alcohol Mycocin A eye drops aqueous micron suspension. Then 50 mg of cyclosporin A and measured amounts of different cyclodextrins (i.e. pure α-cyclodextrin, pure γ-cyclodextrin or a mixture of α-cyclodextrin and γ-cyclodextrin) were added to the solution, and shake until a homogeneous suspension is obtained. The volume was then adjusted to 100.0 ml with a 1.4% (w / v) aqueous solution of polyvinyl alcohol and heated in an autoclave at 121° C. for 20 minutes in a sealed vessel. The suspension was cooled to room temperature under sonication. Then, the suspension was removed from the sonicator and allowed to equilibrate at room temperature with constant stirring for 7 days, protected fr...
Embodiment 3
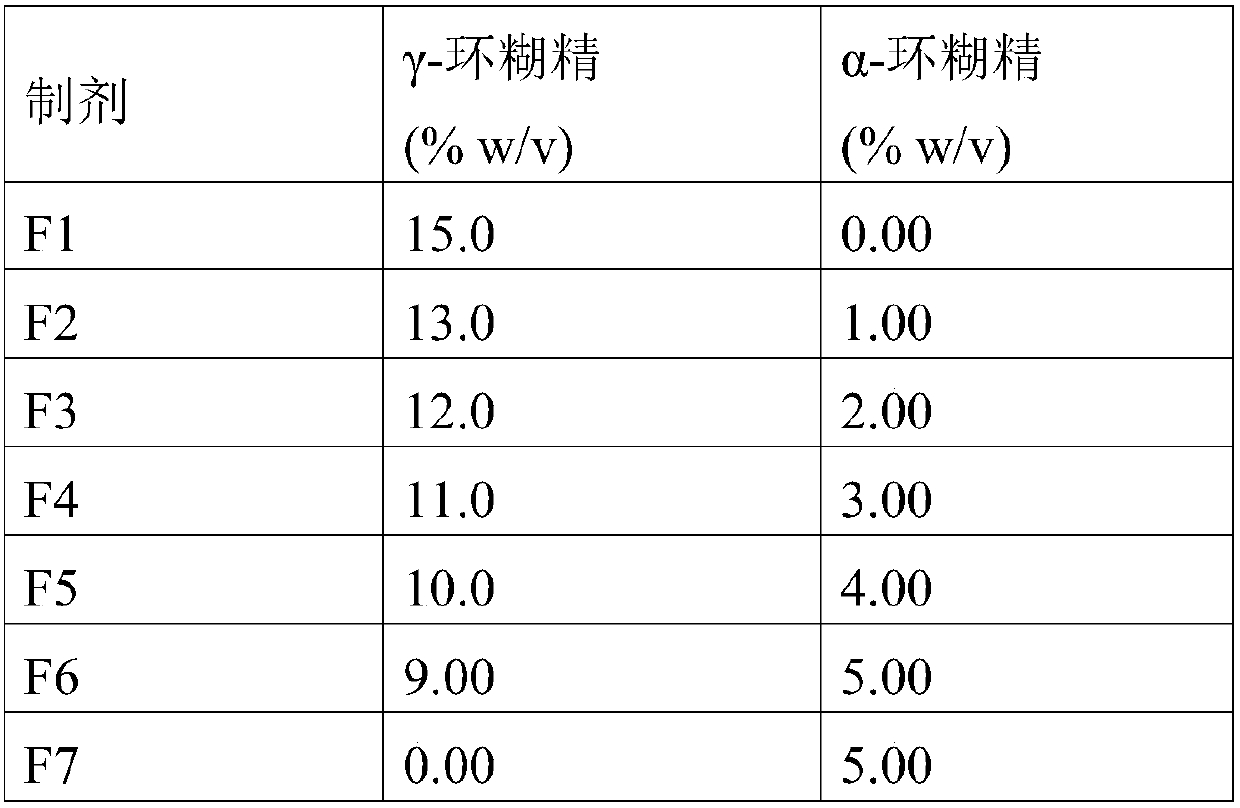
[0111] Particle size characterization of eye drop formulations was performed by dynamic light scattering (DLS). Each formulation was filtered through a 0.45 μm membrane filter (to exclude particles larger than 0.45 μm) prior to measurements performed at 25 °C, 180° scattering angle and 780 nm laser beam, and each measurement was performed in triplicate . Visualizing the particle size using an optical microscope without sample filtration gives a better idea of how many particles are in the suspension and how big they are. Table 6 shows the size distribution data from DLS measurements.
[0112] Table 6
[0113] DLS results for size analysis of cyclosporin A / cyclodextrin complexes for formulations F1-F10, data reported as hydrodynamic diameter (d), population width and intensity distribution (%I) in the nanoscale range
[0114]
[0115]
[0116] DLS measurements of formulations F1 , F2 and F3 gave 2 or 3 size clusters based on the intensity distribution, with an averag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com