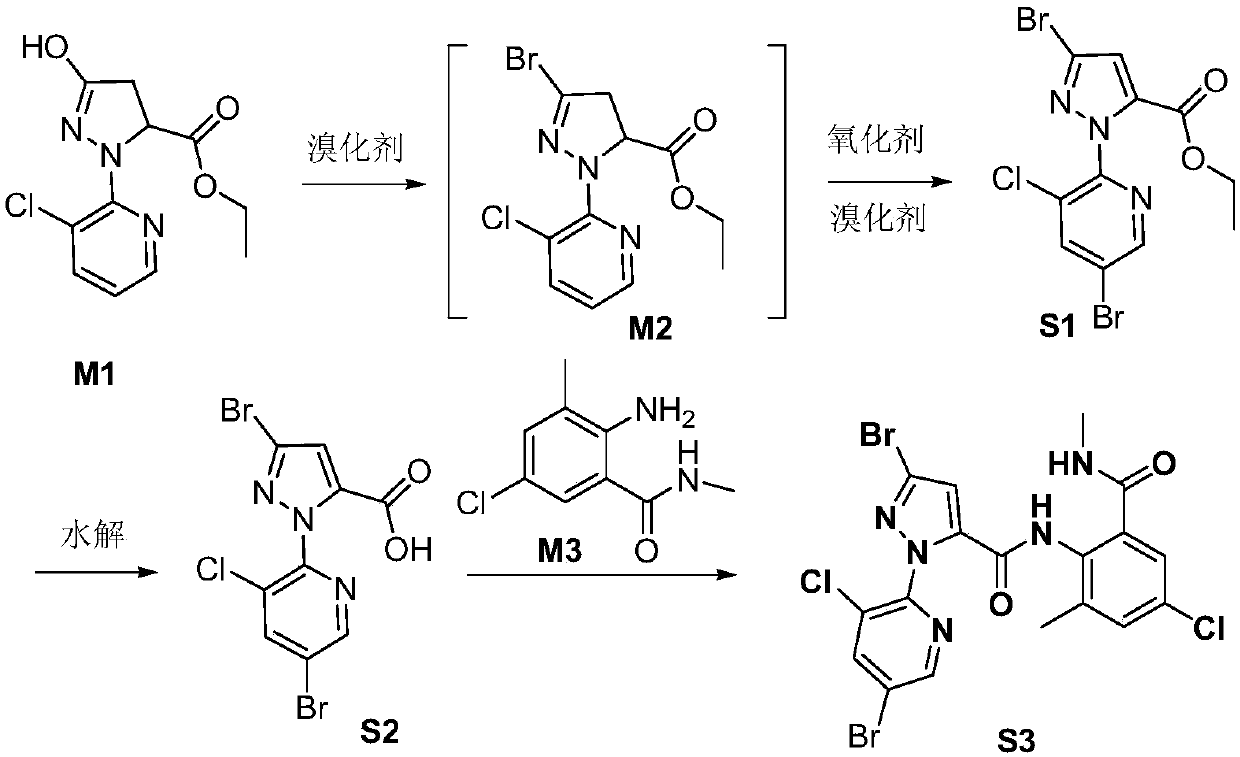
Preparation technology of chlorantraniliprole impurity
A technology for the preparation of chlorantraniliprole and its preparation process, which is applied in the field of the preparation process of chlorantraniliprole impurities, and can solve problems such as consumer hazards, drug properties/toxicological effects, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0115] In the preparation process of the chlorantraniliprole impurity S2, acid-catalyzed or base-catalyzed hydrolysis can be used. Base catalysis is preferred, and optional bases include alkali metal hydroxides, such as NaOH and KOH. The reaction can be carried out in a mixed system of water and alcohol.
[0116] A more specific implementation is:
[0117] Step c, mix water, methanol and sodium hydroxide evenly, and lower the temperature to 15-25°C, add the chlorantraniliprole impurity S1 obtained in step b to it, remove methanol after the reaction is completed, add water, and use acetic acid for the water phase Extract with ethyl ester, then adjust the pH of the aqueous phase to 2-3, stir, filter, and dry to obtain the impurity S2 of chlorantraniliprole.
[0118] Wherein, in the above-mentioned process for preparing the chlorantraniliprole impurity, in step c, the molar ratio of the amount of sodium hydroxide to the added amount of the chlorantraniliprole impurity S1 is 1-3...
Embodiment 1
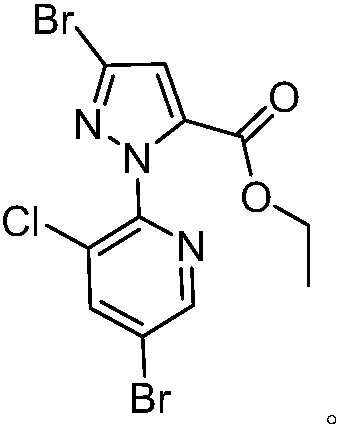
[0141] Example 1: Preparation of Chlorantraniliprole Impurity S1
[0142] (1) Step-by-step preparation
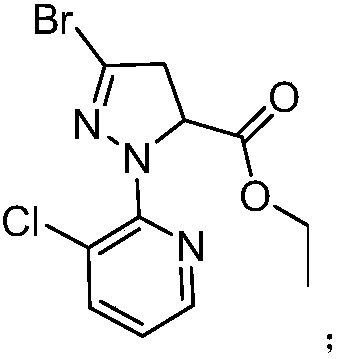
[0143] a. Add 10.0g M1 (0.037mol), 60.0g acetonitrile and 5.3g phosphorus oxybromide (0.018mol) into a 100mL three-necked flask, heat up to 82°C, there is obvious reflux, keep stirring for 2 hours, and naturally cool down to 50°C As follows, a dark green liquid containing flocs was obtained; 2.0 g of diatomaceous earth was added, stirred for 10 min, filtered, the filter cake was washed twice with 2.0 g of acetonitrile, and the organic phase was combined to obtain a reaction solution A; the reaction solution A was added to the saturated Sodium bicarbonate aqueous solution was extracted with dichloromethane, and the organic phase was concentrated under reduced pressure to obtain 10.3 g of M2 with a yield of 97.3% and an HPLC purity greater than 99%.
[0144] b. Take 10.3g (0.036mol) of M2 obtained in step a, 60.0g acetonitrile, 5.3g phosphorus oxybromide (0.018mol), and 15.0...
Embodiment 2
[0149] Embodiment 2: Preparation of chlorantraniliprole impurity S2
[0150] Add 10.0g of water, 15.0g of methanol and 0.6g of sodium hydroxide (0.015mol) into a 100mL single-necked bottle, stir, and when the temperature drops to room temperature, add 5.0g of chlorantraniliprole impurity S1 (0.012mol), and react for 3h , remove most of the methanol under reduced pressure, add 5.0g of water, extract twice with 5g of ethyl acetate, slowly add 2.7g of 30% hydrochloric acid solution dropwise to the water phase, adjust to pH=2~3, stir for 1h, filter, vacuum at 55°C After oven drying for 2 hours, 4.2 g of white solid chlorantraniliprole impurity S3 was obtained, with a yield of 90.2%, HPLC: 96.5%.
[0151] The hydrogen spectrum of the obtained chlorantraniliprole impurity S2 of the present embodiment is: 1 H NMR (400MHz, DMSO-d 6 ): δ8.60(d, J=2.1Hz, 1H), 8.48(d, J=2.1Hz, 1H), 6.51(s, 1H), 13.95(br, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com