All-inorganic lead halogen perovskite nano composite luminous material and preparation method and application
A nanocomposite and luminescent material technology, applied in luminescent materials, nano optics, and material analysis through optical means, can solve the problems of fluorescence quenching and poor thermal stability, and achieve high repetition rate, water resistance and heat resistance. Enhanced performance and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example uses a one-step dissolution-precipitation method to synthesize Cs at room temperature 4 PbBr 6 -CsPbBr 3 -imidazole nanocomposite luminescent material, specifically comprising the following steps:
[0034] (1) Weigh the appropriate metal halide salts CsBr and PbBr according to the molar ratio of 0.4 mmol: 0.1 mmol 2 , 8.2 mg of dimethylimidazole as a chelating agent, 0.5 mL of oleylamine and 0.2 mL of oleic acid as a surfactant, were added together in 10 mL of DMF solution, at room temperature (25 ° C), with a speed of 1000 r / min strong Stir magnetically for 24 hours until all reactants are completely dissolved to form a uniform mixed solution as a reaction precursor;
[0035] (2) Quickly add 0.2 mL of the precursor solution in step (1) to 5 mL of toluene solution, and rapidly magnetically stir at room temperature for 3 minutes at a speed of 500-1500 r / min, and react to form a lead-containing all-inorganic perovskite Complex solution;
[0036] (3) The ...
Embodiment 2
[0040] Similar to Example 1, the difference is that the Cs in Example 1 + and Pb 2+ Keep the molar ratio 4:1 unchanged, add CsCl and PbCl 2 , so that the molar ratio of Br:Cl is 1:1, the Cs of the core-shell coating structure can be obtained 4 PbBr 3 Cl 3 -CsPbBr 1.5 Cl 1.5 -Imidazole nanocomposite luminescent material.
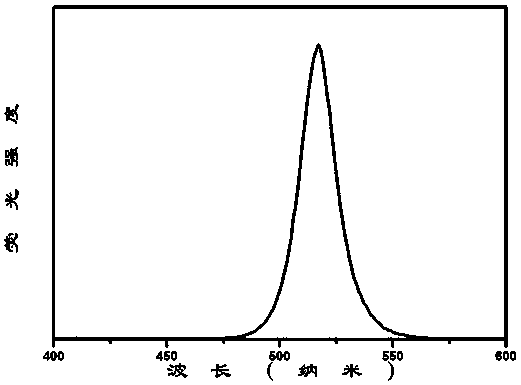
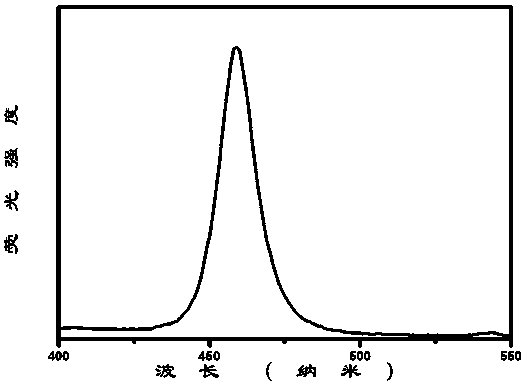
[0041] image 3 for Cs 4 PbBr 3 Cl 3 -CsPbBr 1.5 Cl 1.5 - Emission spectrum of imidazole nanocomposite luminescent material under excitation at 365 nm. It can be seen that: 50% of Br - By Cl - After substitution, the luminescent position of the nanocomposite luminescent material blue-shifts from 516nm to 458nm, showing strong blue emission with a half-maximum width of 16nm and good color purity, and the corresponding CsPbBr 1.5 Cl 1.5 The luminescent properties of the nanocrystal solutions remain consistent.
Embodiment 3
[0043] Similar to Example 2, the difference is that CsCl and PbCl in Example 2 2 Changed to CsI and PbI 2 , both can obtain the Cs of the core-shell coating structure 4 PbBr 3 I 3 -CsPbBr 1.5 I 1.5 -Imidazole nanocomposite luminescent material.
[0044] Figure 4 for Cs 4 PbBr 3 I 3 -CsPbBr 1.5 I 1.5 - Emission spectrum of imidazole nanocomposite luminescent material under excitation at 365 nm. It can be seen that: 50% of Br - By I - After substitution, the luminescent position of the nanocomposite luminescent material red shifts from 516nm to 625nm, showing strong red emission with a half-maximum width of 55nm and good color purity, and the corresponding CsPbBr 1.5 I 1.5 The luminescent properties of the nanocrystal solutions remain consistent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Half width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com