Primers, probes and kits for detection of hepatitis A and hepatitis E virus
A technology for hepatitis E virus and hepatitis A virus, which is applied in the field of primer probe sets for detecting hepatitis A and hepatitis E virus, and can solve problems such as inability to detect hepatitis A and hepatitis E.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] This example is used to illustrate Hepatitis A and Hepatitis E virus-specific primer probe verification tests.
[0063] (1) Synthesis of primers and probes
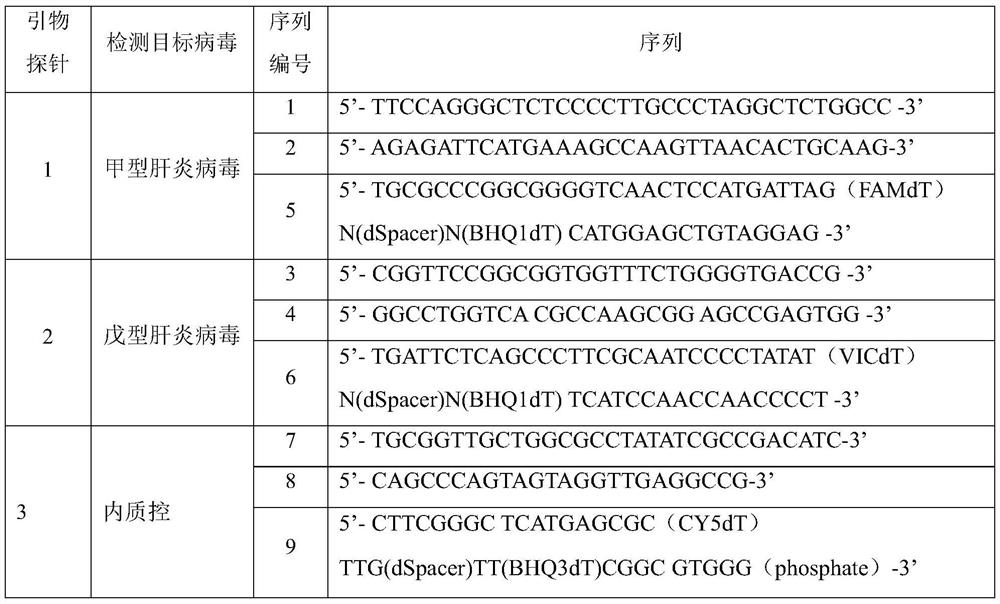
[0064] A reagent company was commissioned to synthesize primer-probe sets 1-3 shown in Table 2.
[0065] Table 2
[0066]
Embodiment 2
[0067] Embodiment 2 specificity verification:
[0068] Select the following samples as simulated interference samples: Norovirus (from National CDC, 2001), Hepatitis B virus (from National CDC, 2005), Hepatitis C virus (from National CDC, 2006), Salmonella (from National Culture Collection Center, CVCC3949), Shigella (from National Culture Collection, CVCC3914), Staphylococcus aureus (from National Culture Collection, CVCC1882), Bacillus cereus (from National Culture Collection, CVCC1782), Listeria monocytogenes (from the National Culture Collection, CVCC1345), Yersinia enterocolitica (from the National Culture Collection, CVCC1365), Enterobacter sakazakii (from the National Culture Collection , CVCC1768), Escherichia coli (from National Culture Collection, CVCC2356), Vibrio cholerae (from National Culture Collection, CVCC3467), Escherichia coli O157 (from National Culture Collection, CVCC7389), Aeromonas hydrophila The nucleic acid of bacteria (from the National Culture Coll...
Embodiment 3
[0072] Embodiment 3 minimum detection limit verification:
[0073] Use a concentration of 10 4 The copies / μL of hepatitis A virus and hepatitis E virus nucleic acid were mixed in equal proportions as a template, and the gradient was diluted to 10 3 copies / μL, 10 2 copies / μL, 10 copies / μL, L, and 1 copy / μL. The verification was carried out according to the reaction procedure of the above reaction system. System preparation and RPA reaction conditions are the same as those in Example 2 for specificity evaluation. In the reaction system, the concentration of the virus was 5×10 4 copy / system, 5×10 3 copy / system, 5×10 2 copies / system, 50 copies / system, 10 copies / μL and 5 copies / system.
[0074] Judgment of RPA reaction results: blank control, IAC and negative control are established, otherwise the experimental results will be considered invalid; if the FAM channel has an amplification curve, the primer pair of SEQ ID NO.1-2 and the probe of SEQ ID NO.5 can be detected If th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com