Polysubstituted chiral (1-ethoxy)benzene and asymmetric synthesis method thereof
A synthetic method and multi-substitution technology, which is applied in the field of asymmetric synthesis of multi-substituted chiral benzene, can solve the problems of diacetylbenzene or triacetylbenzene raw materials are difficult to obtain and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Asymmetric synthesis of (S, S)-1,3-bis(1-hydroxyethyl)benzene
[0038]
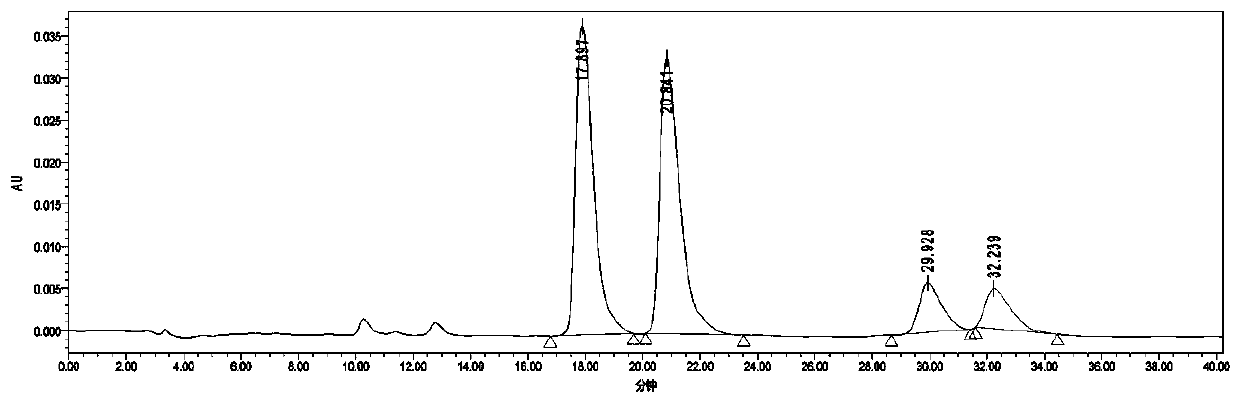
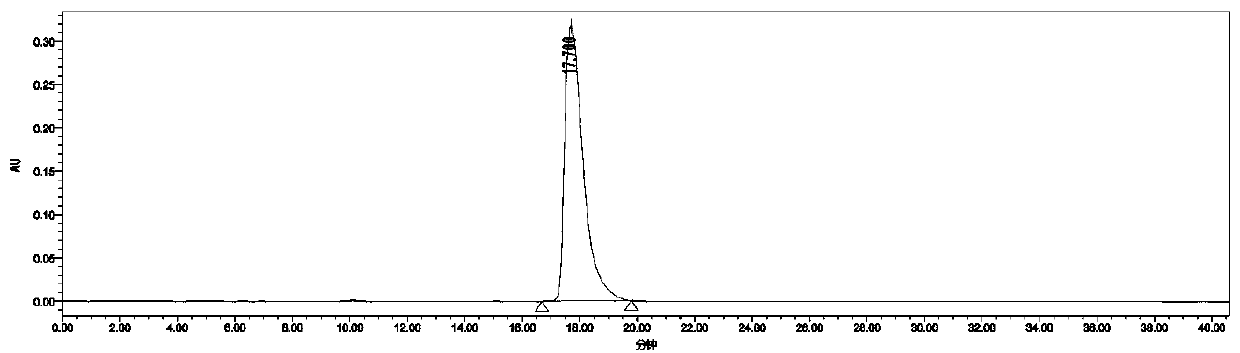
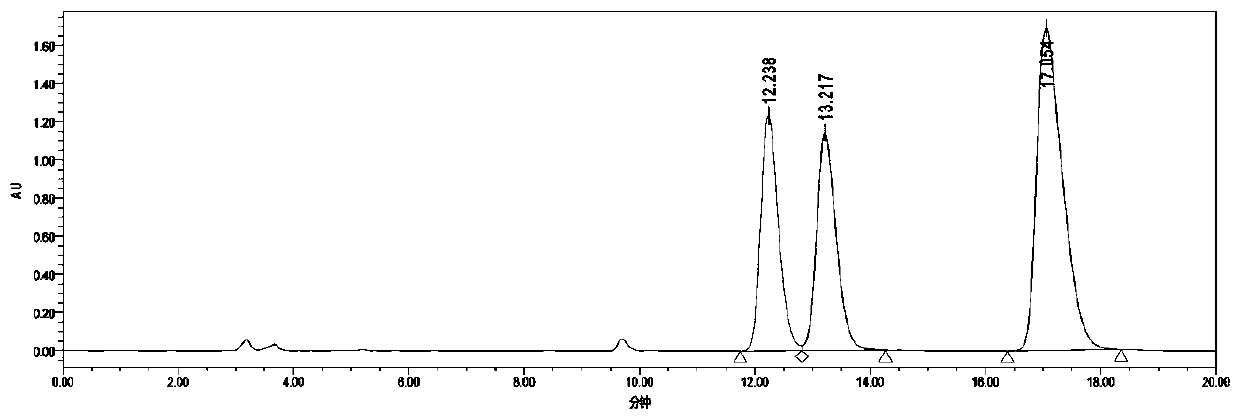
[0039] Add 0.5 mmol of m-diethynylbenzene into the test tube, followed by adding CF 3 SO 3 H (40mol%, 18uL), H 2 O(4 equvi., 40uL), CF 3 CH 2 OH (1mL), after reacting at 60°C for 48h, add 0.005mmol catalyst C, KOH (0.5mmol, 28mg), put the reaction test tube in a high-pressure reactor, replace it three times, then fill it with 4Mpa hydrogen, and react at 40°C for 24 Hour. After the reaction, wash with water, extract the aqueous phase with ethyl acetate 3 times, combine the organic phases and concentrate to dryness, the isolated yield: 86% (petroleum ether: ethyl acetate = 5:1) HPLC assay product (S, S)- The asymmetric synthesis of 1,3-di(1-hydroxyethyl)benzene has an enantiomeric excess value of 99%, a diastereomeric excess value of 99%, and HPLC separation conditions: chiral column Daicel OJ-H column , Mobile phase: n-hexane / isopropanol=90:10 (volume ratio), flow velocity: 1.0 m...
Embodiment 2
[0044] Example 2: Asymmetric synthesis of (S, S)-1,3-bis(1-hydroxyethyl)benzene
[0045]
[0046] Add 0.5 mmol of m-diethynylbenzene into the test tube, followed by adding CF 3 SO 3 H (40mol%, 18uL), H 2 O(4 equvi., 40uL), CF 3 CH 2 OH (1mL), after reacting at 60°C for 48h, add 0.005mmol catalyst E, KOH (0.5mmol, 28mg), put the reaction test tube in a high-pressure reactor, replace it three times, then fill it with 4Mpa hydrogen, and react at 40°C for 24 Hour. After the reaction, wash with water, extract the aqueous phase with ethyl acetate 3 times, combine the organic phases and concentrate to dryness, the isolated yield: 62% (petroleum ether: ethyl acetate = 5:1) HPLC assay product (S, S)- The asymmetric synthesis of 1,3-bis(1-hydroxyethyl)benzene gave an enantiomeric excess of 89% and a diastereomeric excess of 91%.
Embodiment 3
[0047] Example 3: Asymmetric synthesis of (S, S)-1,3-bis(1-hydroxyethyl)benzene
[0048]
[0049] Add 0.5 mmol of m-diethynylbenzene into the test tube, followed by adding CF 3 SO 3 H (40mol%, 18uL), H 2 O(4 equvi., 40uL), CF 3 CH 2 OH (1mL), reacted at 60°C for 48h, added 0.005mmol of catalyst F, KOH (0.5mmol, 28mg), placed the reaction test tube in a high-pressure reactor, replaced 3 times, then filled with 4Mpa hydrogen, and reacted at 40°C for 24 Hour. After the reaction, wash with water, extract the aqueous phase with ethyl acetate 3 times, combine the organic phases and concentrate to dryness, the isolated yield: 71% (petroleum ether: ethyl acetate = 5:1) HPLC assay product (S, S)- The asymmetric synthesis of 1,3-bis(1-hydroxyethyl)benzene gave an enantiomeric excess of 83% and a diastereomeric excess of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com