Novel application of stem cells and secretions thereof in treatment of skin burns and scalds
A stem cell, burn and scald technology, applied in the field of biomedicine, can solve problems that need to be developed, and achieve the effect of promoting proliferation, regulating cell proliferation and differentiation, and promoting the proliferation of wound epidermal cells and dermal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Obtaining and Identification of Exosomes
[0055] 1. Obtaining exosomes
[0056] (1) Provide mesenchymal stem cells
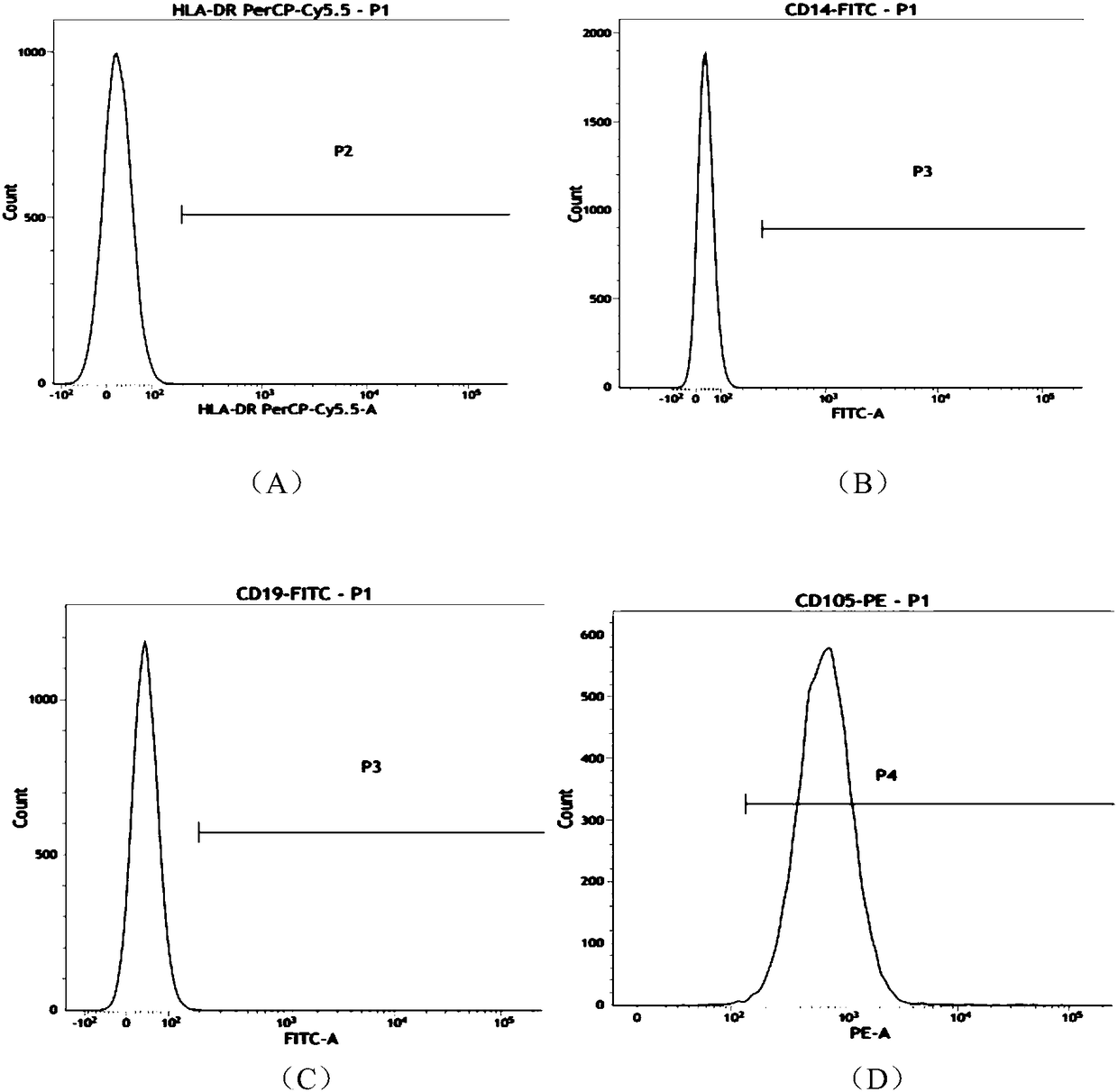
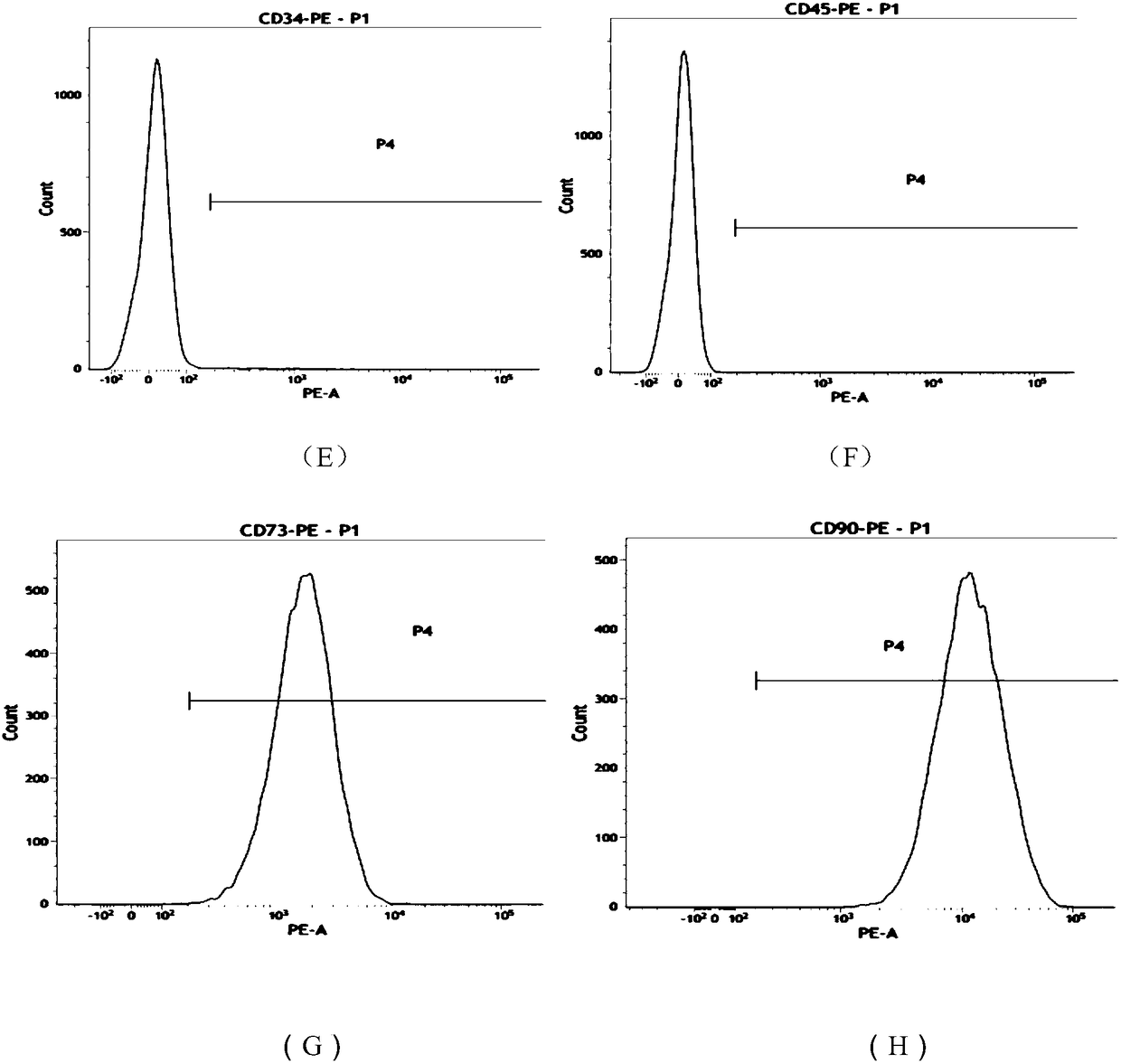
[0057] The morphology of umbilical cord mesenchymal stem cells figure 1 As shown, the mesenchymal stem cells in a good state of expansion and proliferation were taken, and the cells were digested into single cells with TrypLE Express digestive enzyme; then washed with PBS containing 1% BSA, and prepared containing 5×10 6 A single cell suspension of umbilical cord mesenchymal stem cells was labeled with CD90, CD73, CD105, CD34, CD45, CD14, CD19, and HLA-CR flow-type antibodies (BD Company, the United States), and the amount added to each tube was 5 μl at room temperature. Incubate with light for 20 minutes; wash 2 times with PBS containing 1% BSA, resuspend the cells to 400 μl, and perform flow cytometry, the results are as follows: figure 2 As shown, wherein (A) to (H) are HLA-CR, CD14, CD19, CD105, CD34, CD45, CD73, CD90 antibodies respect...
Embodiment 2
[0066] Embodiment 2 Preparation of the second reagent
[0067] (1) Soak fresh oxtail in 75% alcohol for 10 minutes, then rinse repeatedly with sterile saline;
[0068] (2) peel off the tail tendon, remove the sarcolemma and fascia, and place them in a sterile plate;
[0069] (3) Shred the tail tendon, immerse in 0.05mol / L acetic acid solution, and shake at 4°C for 48 hours;
[0070] (4) Move it into a pulverizer for pulverization, and then put it into a refrigerator at 4°C for secondary expansion;
[0071] (5) After 24 hours, filter with a 200-mesh sieve, collect the filtrate, and obtain a collagen solution;
[0072] (6) In a 6-well plate, add 5ml of collagen solution to each well to spread it in the well;
[0073] (7) Pre-freeze the orifice plate in a -30°C refrigerator for 6 hours, and then vacuum-dry it for 18-24 hours to obtain a collagen sponge;
[0074] (8) Place the collagen sponge in a 10 cm petri dish, add 30 mL of 0.25% glutaraldehyde solution to the petri dish f...
Embodiment 3
[0080] Example 3 in vitro induction of mesenchymal stem cells to differentiate into epidermal cells
[0081] 1. Differentiation of mesenchymal stem cells into epidermal cells in vitro
[0082] Cells were planted at a density of 6×10 on a 6-well plate pre-coated with Matrigel (BD Company, USA). 4 cells / cm 2 For umbilical cord mesenchymal stem cells, add 2ml of low-sugar DMEM medium containing 10% fetal bovine serum to each well, at 37°C, 5% CO 2 The cells were cultured in an incubator until the cells reached 50% confluency, and then 2 ml of epidermis induction conditioned medium was used for induction for 7 days, and the medium was changed every other day in half.
[0083] Epidermal induction conditioned medium formula: (DMEM:DF12=1:1 (purchased from Sigma), 20ng / ml epidermal growth factor (purchased from R&D), 15ng / ml basic fibroblast growth factor (purchased from R&D), 1% insulin-transferrin-selenium complex (purchased from Sigma), 0.1 μM dexamethasone (purchased from Sigm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epidermal growth factor | aaaaa | aaaaa |
| Epidermal growth factor | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com