Novel Schiff base type amine antioxidant as well as preparation method and application thereof
A technology of Schiff base amine and antioxidant, applied in the field of antioxidants, can solve problems such as comprehensive performance needs to be further improved, and achieve the effects of excellent thermal stability, simple post-processing, and simple and easy operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] In some preferred embodiments, the preparation method comprises: taking aldehydes or ketones, diphenylamine and / or diphenylamine derivatives and / or phenothiazines and / or phenothiazine derivatives in the presence of a catalyst react in a liquid phase reaction medium to obtain the Schiff base amine antioxidant.
[0055] For example, in a more specific embodiment, the preparation method may include: reactant A and reactant B exist in catalyst C, and react in optional liquid phase reaction medium D, thereby forming suitable, especially suitable As the reaction product of antioxidants, namely the aforementioned Schiff base amine antioxidants.
[0056] For example, the following components can be used as reactants to form the products of the invention:
[0057] Reactant A: aldehydes or ketones include alkyl aldehydes or ketones and / or aromatic aldehydes or ketones; preferably, the reactant A is selected from aldehydes, which can be non-limitingly selected from various types ...
Embodiment 1
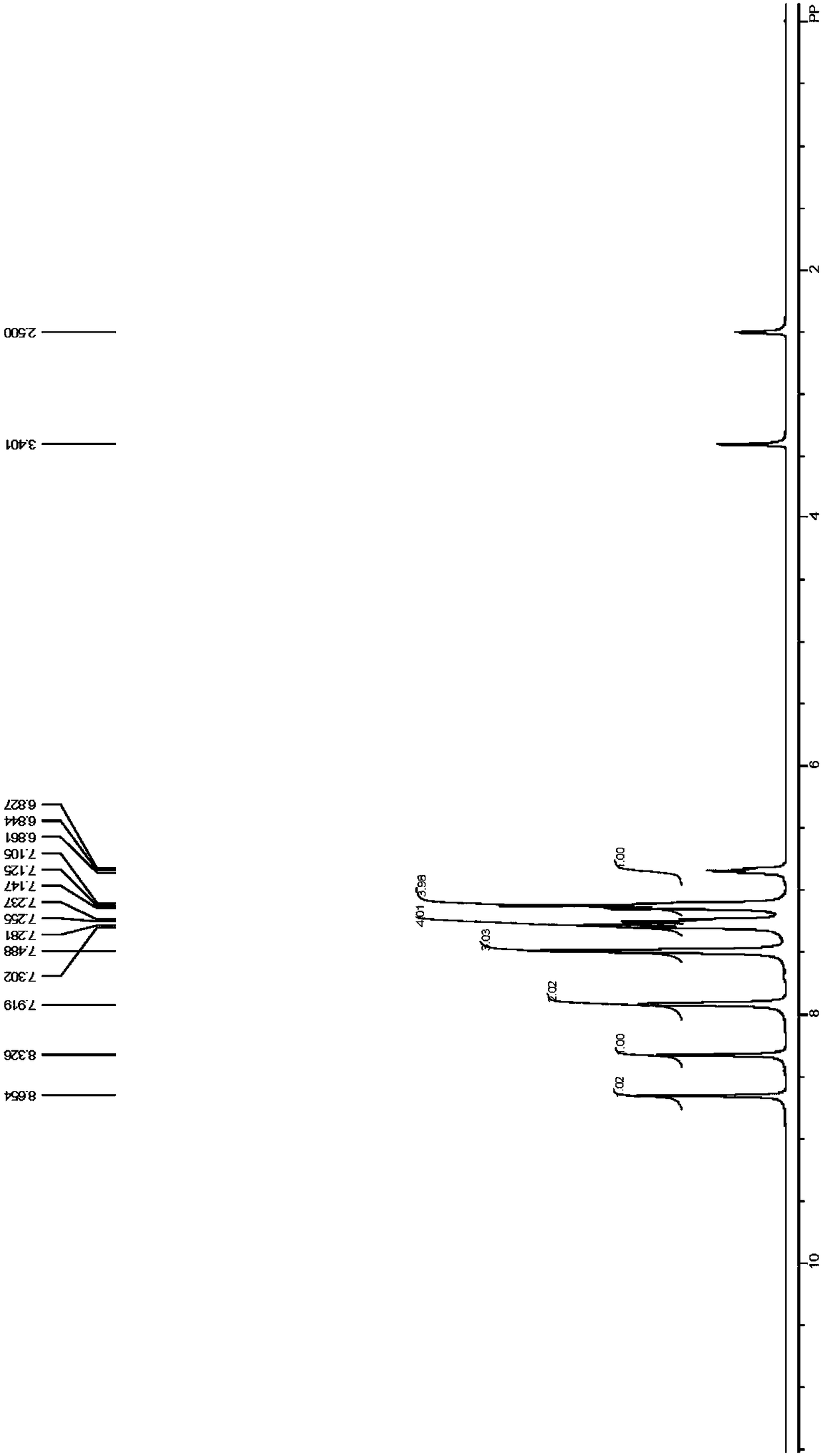
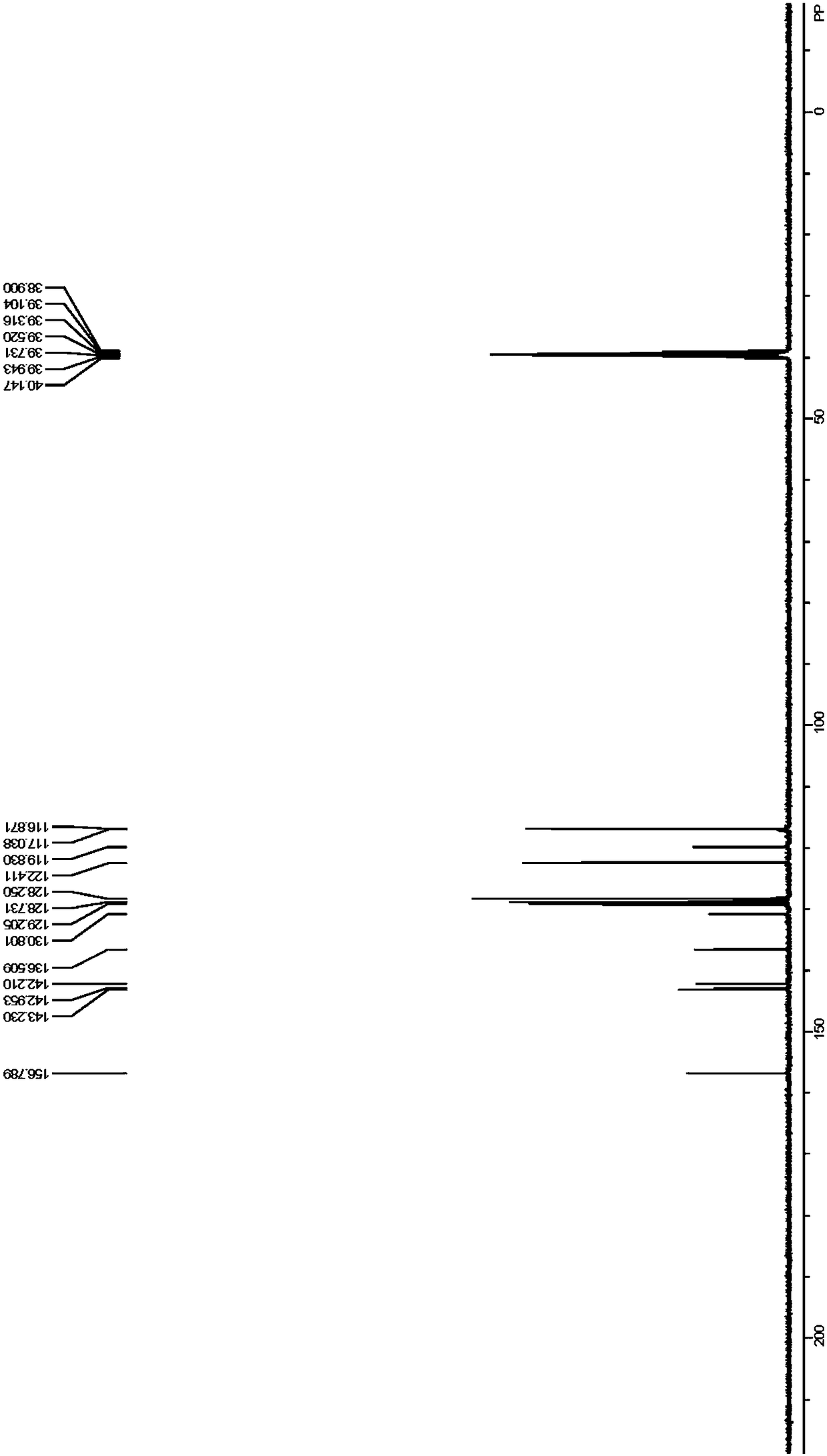
[0078] Embodiment 1 Benzaldehyde 5g-7.5g, 4-aminodiphenylamine 12g-15g and sulfuric acid 0.1g-1g are dissolved in about 100mL tetrahydrofuran, join in the thick-walled pressure-resistant glass bottle that has magneton, at reaction temperature React under the condition of 50°C-110°C for 3h-8h. After the reaction is over, cool the reaction mixture to room temperature, remove the solvent under reduced pressure, and recrystallize to obtain the target product. 1 H NMR spectrum, 13 C NMR spectra can be found separately Figure 1-Figure 2 . 1 H NMR (400MHz, DMSO-d 6 )δ6.85(t,J=6.8Hz,1H),7.11-7.15(m,4H),7.24-7.30(m,4H),7.49(s,3H),7.92(s,2H),8.33(s ,1H),8.66(s,1H). 13 C NMR (100MHz, DMSO-d 6 )δ116.87, 117.04, 119.83, 122.41, 128.25, 128.73, 129.21, 130.80, 136.51, 142.21, 142.95, 143.23, 156.79. IR (film): 745, 824, 1315, 1450, 1508, 158, 158, The average yield is more than 70%, the preferred product yield is greater than 95%, and the product purity is greater than 98%.
Embodiment 2
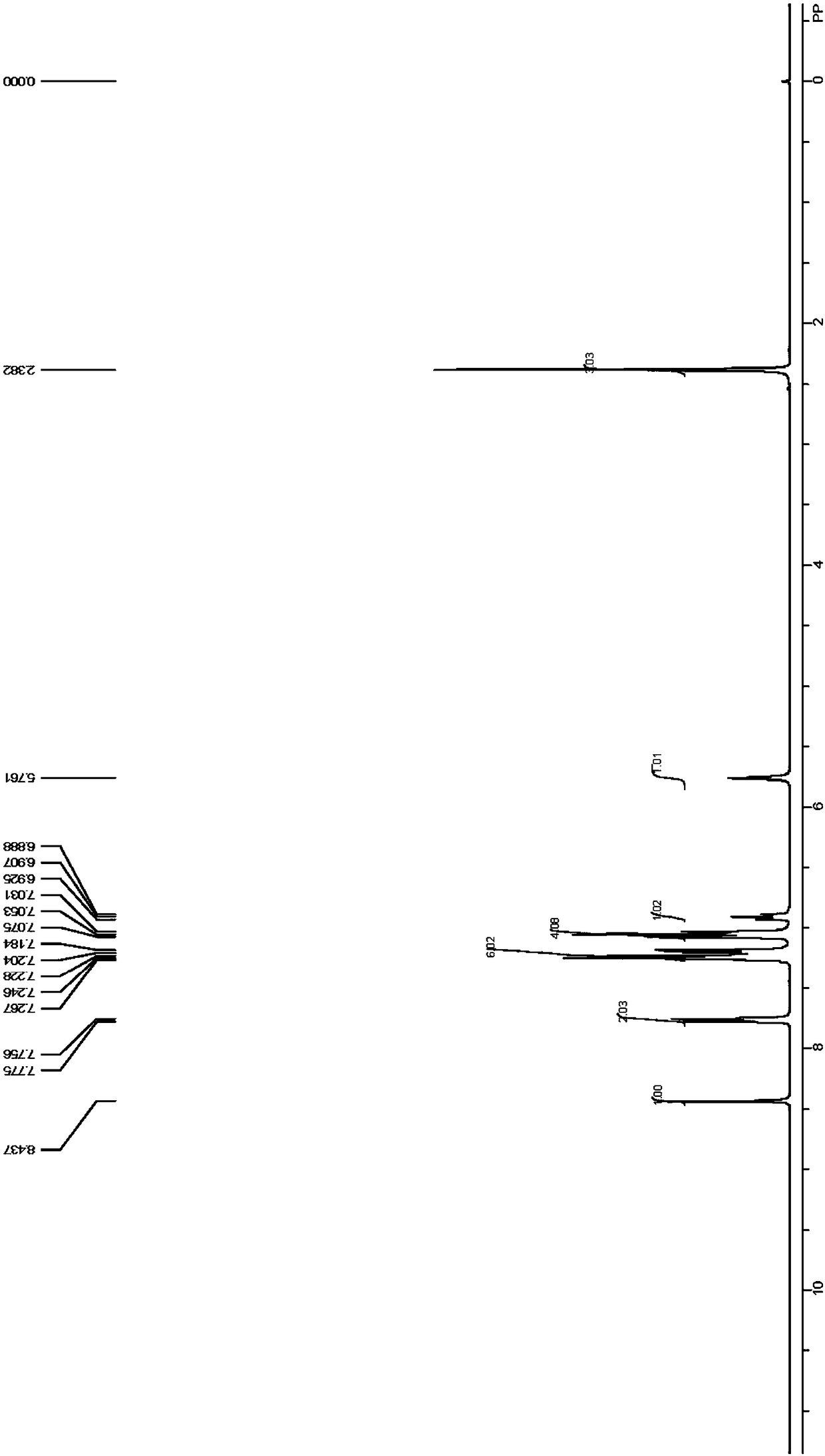
[0079] Example 2 Dissolve 6g-9g of p-tolualdehyde, 14g-17g of 4-aminodiphenylamine and 0.1g-2g of hydrochloric acid in about 100mL of chloroform, and add them to a thick-walled pressure-resistant glass bottle with a magnet , react at a reaction temperature of 60°C-100°C for 6h-10h, after the reaction is over, cool the reaction mixture to room temperature, remove the solvent under reduced pressure, and recrystallize to obtain the target product. 1 H NMR spectrum, 13 C NMR spectra can be found separately Figure 3-Figure 4 . 1 H NMR (400MHz, CDCl 3 )δ2.38(s,3H),5.76(s,1H),6.89-6.93(m,1H),7.05(d,J=8Hz,4H),7.18-7.27(m,6H),7.77(d, J=7.6Hz,2H),8.44(s,1H). 13 C NMR (100MHz, CDCl 3 )δ21.54,117.54,118.48,120.83,122.14,128.52,129.30,129.42,133.87,141.33,143.13,145.18,157.89.IR(film):686,738,826,1333,1348,1498,1530,1587,1603,1623,2920, 3034, 3398. The average product yield in this example is above 50%, the preferred product yield is greater than 75%, and the product purity is great...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com