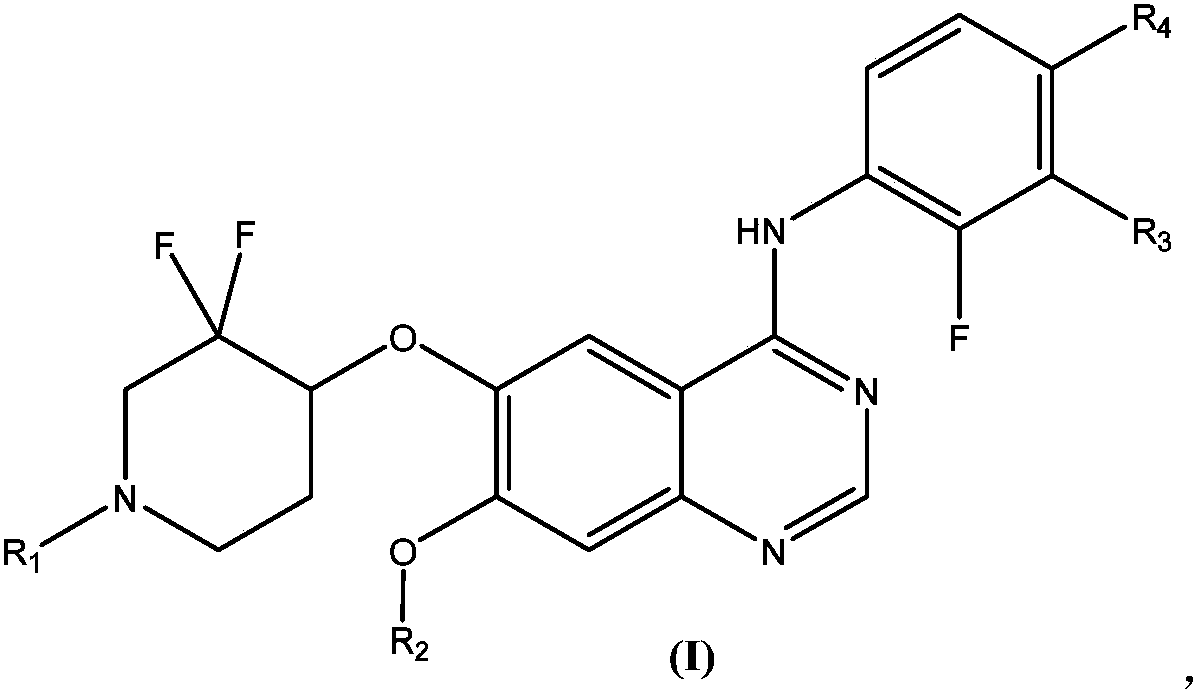
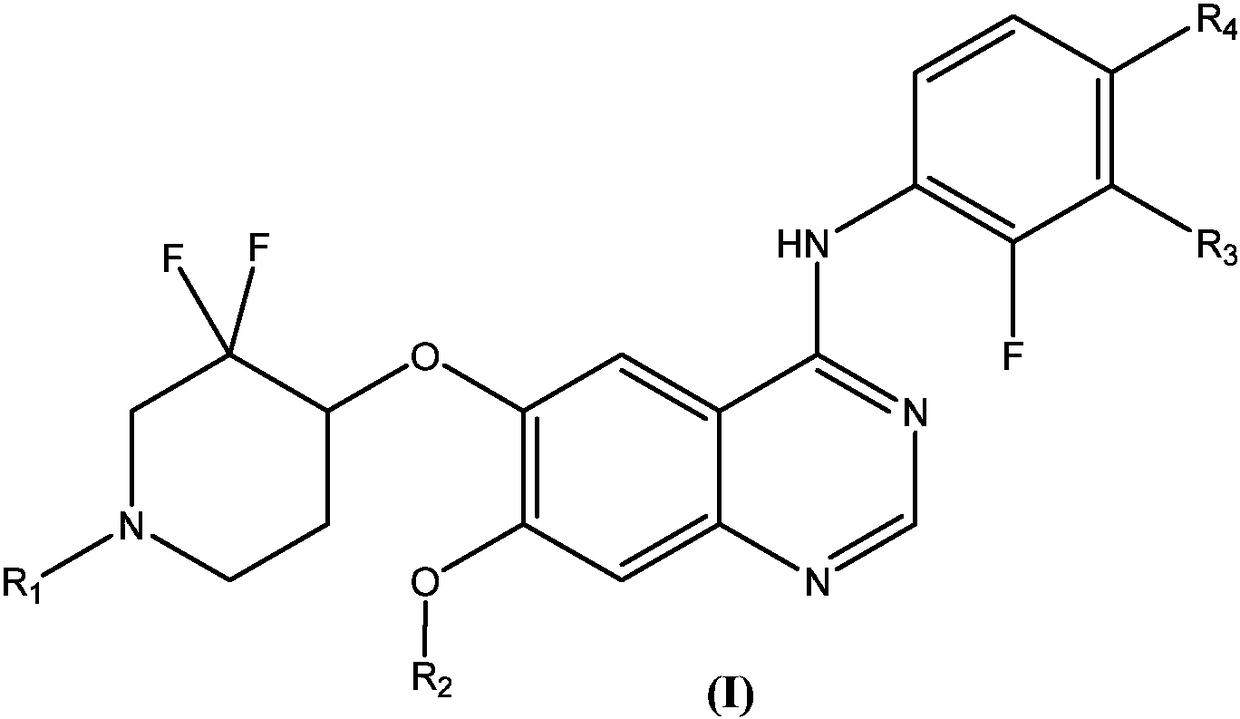
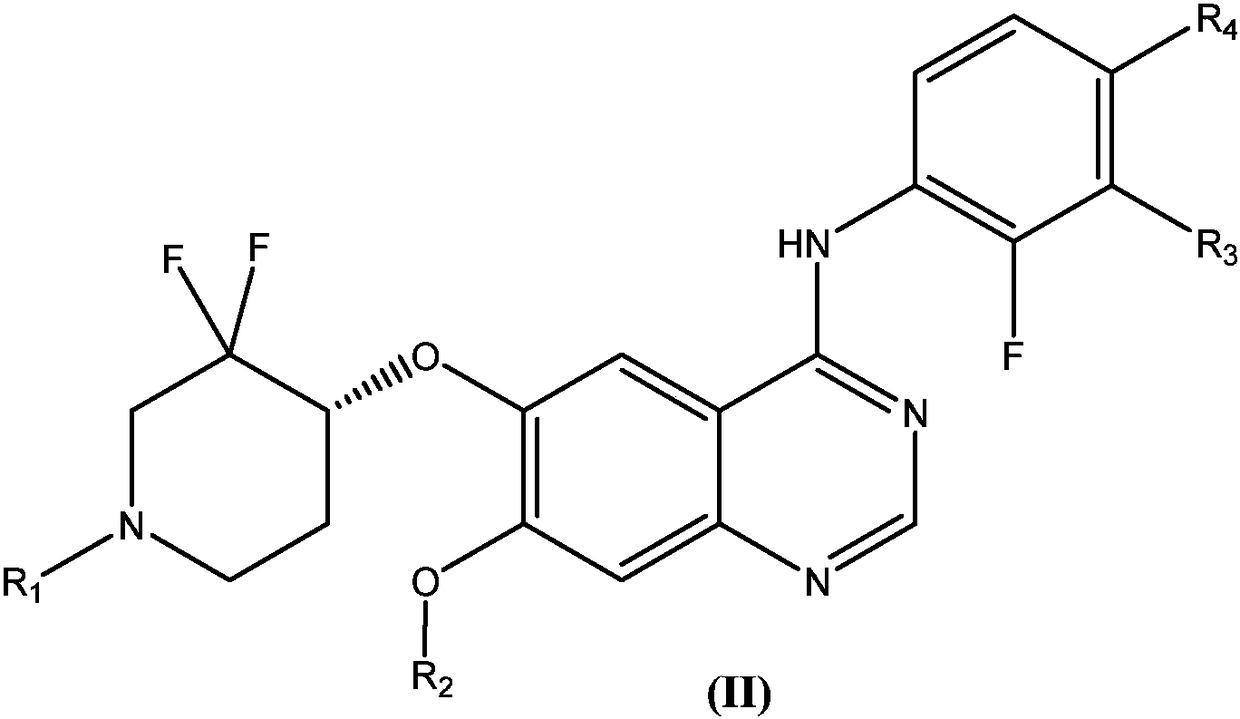
Substituted quinazoline compound capable of crossing blood-brain barrier
A compound, the technology of deuterated alkyl, applied in the field of substituted quinazoline compounds, can solve the problems of limited therapeutic effect, etc., to reduce drug resistance, improve tablet intake compliance, good pharmacokinetics and high biological activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245] Synthesis of intermediate 5-fluoro-4-methoxy-2-nitrobenzonitrile A6 and 1-bromo-5-fluoro-4-(deuteromethoxy)-2-nitrobenzene C1, the synthetic route is as follows :
[0246] Step 1: To a solution of A1 (2.0 g, 10.5 mmol) and triethylamine (1.3 g, 12.6 mmol) in dichloromethane (10 mL) was added dropwise ethyl chloroformate in dichloromethane (3 mL) at 0 °C Ester (1.4 g, 12.6 mmol) solution. The reaction mixture was stirred at 0 °C for 1 h and allowed to reach room temperature. The reaction mixture was then washed twice with water. The organic layer was dried over magnesium sulfate and evaporated in vacuo to afford product A2 (2.7 g, 100% yield) as a colorless oil.
[0247]Step 2: To a solution of A2 (2.7 g, 10.3 mmol) in concentrated sulfuric acid (4.6 mL) was added dropwise fuming nitric acid (0.73 mL, 15.5 mmol) at 10°C. After 1 hour, the reaction mixture was poured into ice / water and extracted twice with ethyl acetate. The combined organic layers were washed with...
Embodiment 2
[0268] Synthesis of compounds 7, 23 and 25: (R / S)-nitrogen-(3-chloro-2,4-difluorophenyl)-6-{[3,3-difluoro-1-(oxetane Synthesis of alkane-3-yl)piperidin-4-yl]oxyl group}-7-methoxyquinazolin-4-amine (7) and separation into enantiomerically pure ( R)-nitrogen-(3-chloro-2,4-difluorophenyl)-6-{[3,3-difluoro-1-(oxetane-3-yl)piperidin-4-yl ]oxy}-7-methoxyquinazolin-4-amine (23) and (S)-nitrogen-(3-chloro-2,4-difluorophenyl)-6-{[3,3- Difluoro-1-(oxetane-3-yl)piperidin-4-yl]oxy}-7-methoxyquinazolin-4-amine (25), the synthetic route is as follows:
[0269]
[0270] Step 1: A mixture of A9 (90 mg, 0.21 mmol) and 3-chloro-2-,4-difluoroaniline (38 mg, 0.23 mmol) in acetic acid (2 mL) was stirred at 120° C. for 3 hours under nitrogen protection. After cooling, it was treated with saturated sodium bicarbonate solution to pH=8 and extracted with dichloromethane. The organic phase was dried over sodium sulfate and concentrated in vacuo to give a residue which was stirred in hydrochloric ...
Embodiment 3
[0274] Synthesis of Compounds 2, 12 and 14: (R / S)-6-{[3,3-Difluoro-1-(oxetan-3-yl)piperidin-4-yl]oxyl}- Synthesis of nitrogen-(3-ethynyl-2-fluorophenyl)-7-methoxyquinazolin-4-amine 2 and separation into enantiomerically pure (R)-6 by HPLC chiral separation column -{[3,3-Difluoro-1-(oxetane-3-yl)piperidin-4-yl]oxy}-nitrogen-(3-ethynyl-2-fluorophenyl)-7 -Methoxyquinazolin-4-amine (12) and (S)-6-{[3,3-difluoro-1-(oxetan-3-yl)piperidin-4-yl] Oxygen)-nitrogen-(3-ethynyl-2-fluorophenyl)-7-methoxyquinazolin-4-amine (14), the synthetic route is as follows:
[0275]
[0276] Step 1: A mixture of A9 (210 mg, 0.48 mmol) and 2-fluoro-3-((trimethylsilyl)ethynyl)aniline (199 mg, 0.96 mmol) in acetic acid (4.2 mL) at 80 °C Stir under nitrogen for 16 hours. After cooling, it was treated with saturated sodium bicarbonate solution to pH = 8 and extracted with dichloromethane. The organic phase was dried over sodium sulfate and concentrated in vacuo to obtain a residue, which was purified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com