Application of medicinal composition containing eucalyptol, limonene and alpha-pinene in preparing drug for treating upper respiratory bacterial infection
A technology for upper respiratory tract and bacterial infection, which is applied to the application field of a pharmaceutical composition containing eucalyptol, limonene and α-pinene in the preparation of a drug for treating bacterial infection of the upper respiratory tract, achieving a clear chemical structure and composition, and high safety. High, promotes the effect of returning to normal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Nebulized inhaler
[0029] The prescription for aerosol inhalation dosage form is: 7.2g eucalyptol (purity 85.0%), 4.5g limonene (purity 96.0%), 3.82g α-pinene (purity 94.0%) (eucalyptol, α-pinene) The weight ratio of the total weight to the limonene is 22:9, and the three components account for 1.55% of the total weight of the prescription), 15.0g poloxamer 407, 5.0g polyoxyethylene sorbitan monooleate, 12.0g chlorine Dilute with sodium chloride, 2.0g vitamin C, 0.1g carbomer, and 0.9% saline to 1000mL.
[0030] Preparation method: First pass the solid excipients through an 80-mesh sieve for later use. Mix eucalyptol, limonene and α-pinene into poloxamer 407 and polyoxyethylene sorbitan monooleate, stir in vacuum for 40 minutes, and add 70% of the prescription amount of physiological saline was stirred for 5 minutes for high-pressure homogenization twice, vitamin C and carbomer were added, potassium dihydrogen phosphate and disodium hydrogen phosphate were added to adjust ...
Embodiment 2
[0032] Enteric-coated soft capsules
[0033] The content of the enteric-coated soft capsule is prescribed as follows: eucalyptol 1.6 kg, limonene 1.05 kg, α-pinene 0.35 kg, and soybean oil 1.0 kg.
[0034] The prescription of the capsule shell of the enteric soft capsule is: 1 part by weight gelatin, 1.1 parts by weight of water, and 0.35 parts by weight of glycerin; the enteric coating is composed of 6 parts by weight of polyacrylic resin II, 12 parts by weight of polyacrylic resin III, and 4.5 parts by weight of castor. Sesame oil, 4.5 parts by weight of polyethylene phthalate, 2 parts by weight of polysorbate 80, and 1.25 parts by weight of polyethylene glycol 6000.
[0035] The content of the enteric soft capsule is prepared by pressing, shaping, and washing the soft capsule, and then directly wrapping a disposable molded polymer enteric coating material outside the capsule shell.
Embodiment 3
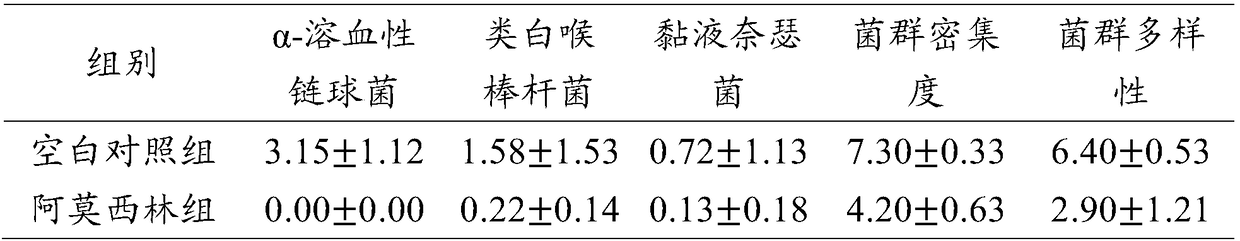
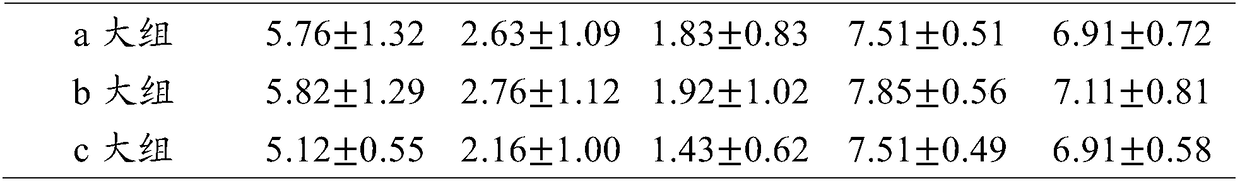
[0037] Restore and regulate the balance of flora
[0038] The composition of the pharmaceutical composition used in this example is as follows (mass ratio of eucalyptol, limonene and α-pinene):
[0039] Group a: Eucalyptus 18.0%-35.0%, Limonene 44.0%-60.0%, α-Pinene 25.0%-30.0%;
[0040] Group b: Eucalyptol 35.0%-55.0%, Limonene 32.0%-44.0%, α-Pinene 10.0%-25.0%;
[0041] Group c: Eucalyptus 55.0%-63.0%, Limonene 15.0%-32.0%, α-Pinene 5.0%-10.0%.
[0042] Take three groups of pharmaceutical compositions from group a. The mass ratios of eucalyptol, limonene and α-pinene in the three groups are as follows:
[0043] (1) Eucalyptus 18.0%, limonene 55.0%, α-pinene 27.0%;
[0044] (2) Eucalyptus 25.0%, limonene 60.0%, α-pinene 25.0%;
[0045] (3) Eucalyptus oil 33.0%, limonene 44.0%, α-pinene 29.0%.
[0046] Take three groups of pharmaceutical compositions from group b. The mass ratios of eucalyptol, limonene and α-pinene in the three groups are as follows:
[0047] (4) Eucalyptus oil 36.0%, limon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com