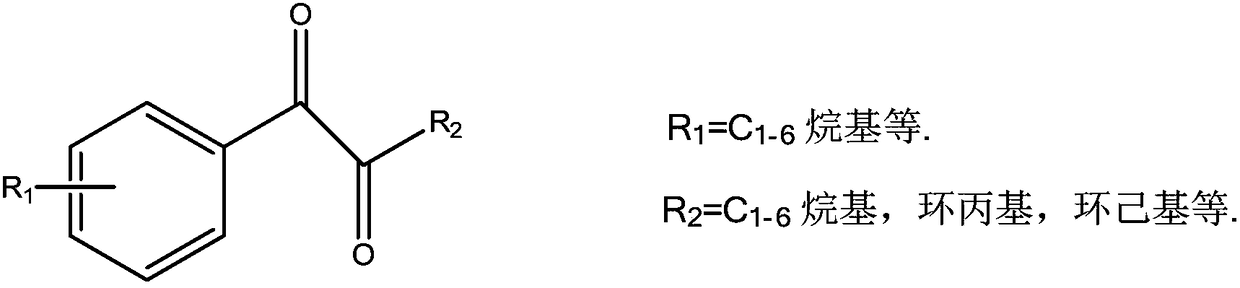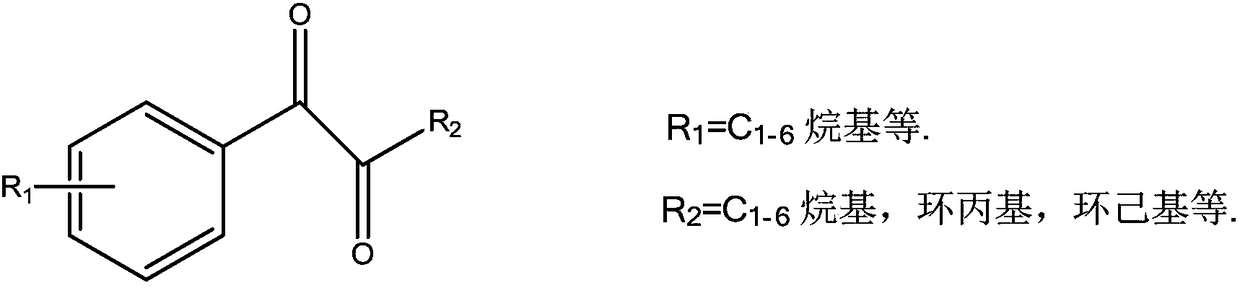1-phenyl-1,2-dione compound LED photoinitiators and synthesis method thereof
A technology of photoinitiator and synthesis method, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor absorption of light energy, poor initiation performance, etc. The effect of an economical production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
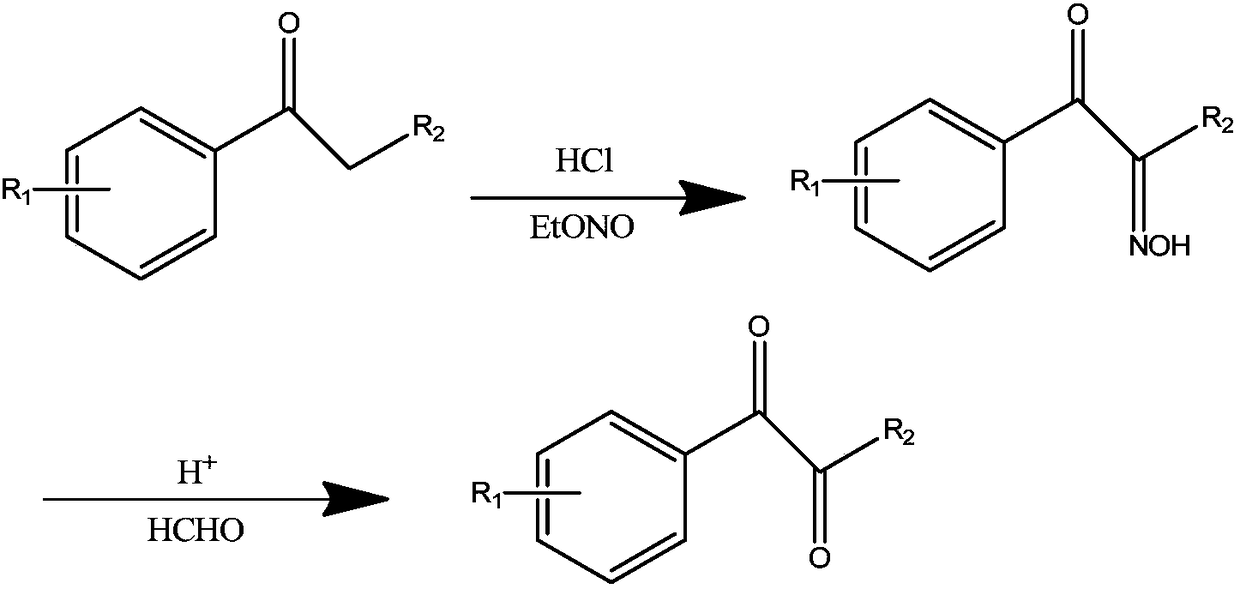
[0022] (1) In the reactor of 2000 liters, add 800kg ethanol and 400kg propiophenone, pass into 78kg dry hydrogen chloride gas under the cooling of ice water, keep passing into 242kg ethyl nitrite at 30-35 ℃, continue to pass through after finishing The temperature was reacted for 2 hours, the reaction was stopped, and the ethanol was distilled off under reduced pressure to obtain a crude product. 700 kg of toluene was added for recrystallization to obtain 310 kg of 1-phenyl 1,2-propanedione-2-oxime with a yield of 63.8%.
[0023] (2) In a 1000-liter reaction kettle, add 200kg of concentrated hydrochloric acid, 200kg of 30% formaldehyde solution and 200kg of ethanol, cool under ice water cooling below 15°C, and add 200kg of 1-phenyl 1,2-propanedione-2 in batches -oxime, stirred at room temperature and reacted for 8 hours after addition, extracted with 400kg of dichloromethane, then washed once with saturated sodium bicarbonate solution and water, removed dichloromethane to obta...
Embodiment 2
[0025] (1) In a 1000 ml reaction flask, add 300 g of ethanol and 148 g of isobutyrophenone, feed 40 g of dry hydrogen chloride gas under ice water cooling, and feed 120 g of ethyl nitrite at 30-35 °C. Continue to react at this temperature for 2 hours, stop the reaction, distill off ethanol under reduced pressure, add 300kg of toluene, wash with 100g of water twice, remove toluene, and obtain 1-phenyl-3-methyl-1,2-butanedione -2-oxime 139g, yield 78.5%. Go directly to the next reaction.
[0026] (2) In a 1000ml reaction flask, add 150g of concentrated hydrochloric acid, 150g of 30% formaldehyde solution and 200kg of ethanol, and add 139g of 1-phenyl-3-methyl-1,2- Butanedione-2-oxime, stirred at room temperature and reacted for 12 hours after the addition, extracted with 200g of dichloromethane, then washed once with saturated sodium bicarbonate solution and water, removed the dichloromethane to obtain the crude product, and distilled under reduced pressure to obtain Yellow li...
Embodiment 3
[0028] (1) In a 1000 ml reaction flask, add 300 g of ethanol and 160 g of phenylcyclopropylmethyl ketone, feed 40 g of dry hydrogen chloride gas under ice water cooling, and feed 120 g of ethyl nitrite at 30-35°C After passing through, continue to react at this temperature for 2 hours, stop the reaction, evaporate ethanol under reduced pressure, add 300kg toluene, wash twice with 100g water respectively, remove toluene, and obtain 1-phenyl-2-cyclopropyl-1, 148 g of 2-propanedione-2-oxime, the yield was 78.3%. Go directly to the next reaction.
[0029] (2) In a 1000ml reaction flask, add 150g of hydrochloric acid, 150g of formaldehyde solution and 150g of ethanol, cool under ice water cooling and add 148g of 1-phenyl-2-cyclopropyl-1,2-propane in batches below 15°C Diketone-2-oxime, stirred at room temperature and reacted for 2 hours after addition, extracted with 300g dichloromethane, then washed once with saturated sodium bicarbonate solution and water, removed dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com