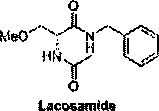High-efficiency racemic lacosamide preparation method
A lacosamide, high-efficiency technology, applied in the field of lacosamide preparation, can solve the problems of complex steps, long route, easy allergies, etc., and achieve the effect of high atom utilization rate, good economic benefit and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
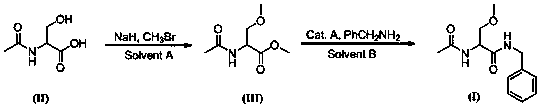
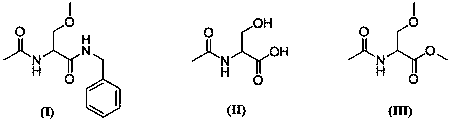
[0028] Example 1: Synthesis of 2-acetylamino-3-methoxypropionic acid methyl ester
[0029] According to the amount ratio of N-acetylserine: bromide: sodium hydride is 1.0:2.0:3.0 feeding; N-acetylserine 44.1g, bromide 57.0g, sodium hydride 36.0g; anhydrous organic solvent A is DMF 660mL, its The volume dosage is 15 times the mass of N-acetylserine (mL / g).
[0030] In a dry and clean reaction flask, protect it with nitrogen gas, slowly add DMF, sodium hydride and N-acetylserine in sequence, stir, cool down to -10~-5°C in an ice-salt bath, slowly introduce methyl bromide, and control the reaction temperature at 0°C the following. After the dropwise addition was completed, the reaction was carried out at 80° C. for 3 hours. The reaction solution was quenched with ice water, extracted with methyl tert-butyl ether, and the organic phase was concentrated under reduced pressure to obtain 36.8 g of methyl 2-acetylamino-3-methoxypropionate intermediate, with a yield of 70.0%.
Embodiment 2
[0032] According to the ratio of N-acetylserine: methyl bromide: sodium hydride is 1.0: 2.0: 2.1 feeding; N-acetylserine 44.1g, methyl bromide 57.0g, sodium hydride 25.2g; anhydrous organic solvent A is DMSO 880mL, its The volume dosage is 20 times the mass of N-acetylserine (mL / g).
[0033] The reaction temperature was 20°C, and the reaction was carried out for 10 hours. Other operations were the same as in Example 1 to obtain 42.5 g of methyl 2-acetylamino-3-methoxypropionate, with a yield of 80.9%.
Embodiment 3
[0035] N-acetylserine: methyl bromide: sodium hydride is 1.0: 2.5: 3.5 according to the amount of material; N-acetylserine 44.1g, methyl bromide 71.2g, sodium hydride 42.0g; anhydrous organic solvent A is N,N- Dimethylacetamide 660mL, its volume is 15 times the mass of N-acetylserine (mL / g).
[0036] The reaction temperature was 40°C, and the reaction was carried out for 8 hours. Other operations were the same as in Example 1 to obtain 42.0 g of methyl 2-acetylamino-3-methoxypropionate, with a yield of 79.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com