A method for extracting and separating amlodipine enantiomers using amino acid ionic liquid
A technology of ionic liquid and amlodipine, which is applied in liquid solution solvent extraction, organic chemical methods, chemical instruments and methods, etc., can solve the problems of high energy consumption, easy pollution of the environment, low selectivity, etc., and achieve good separation effect, The effect of improving separation efficiency and increasing energy demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
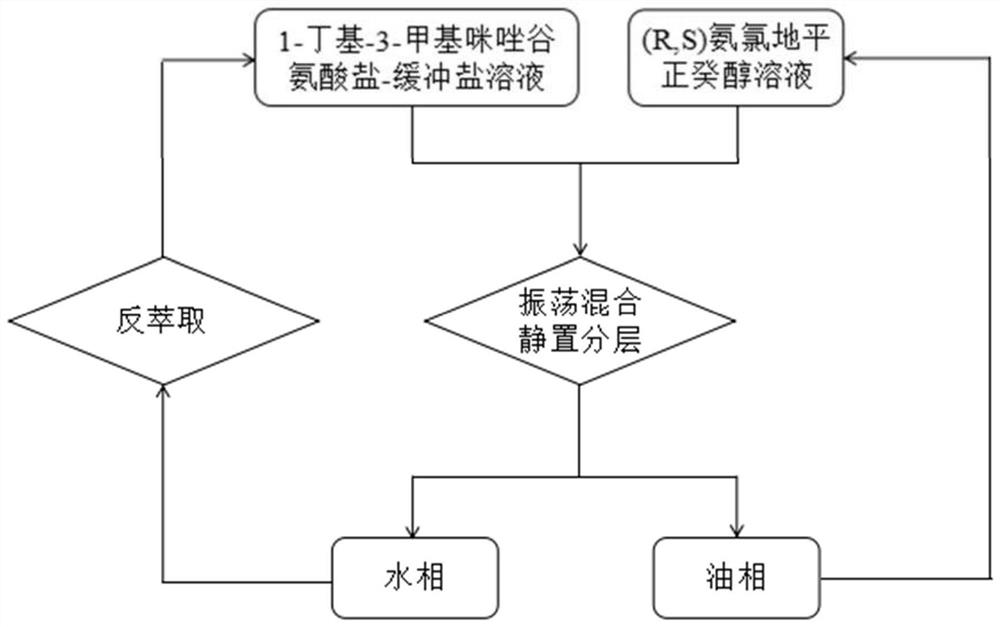
[0019] Such as figure 1 As shown, a certain amount of 1-butyl-3-methylimidazolium glutamate is dissolved in the acetic acid-sodium acetate buffer solution (pH=5.5) with a concentration of 0.01mol / L to make an amino acid ionic liquid with a concentration of 0.005mol / L water phase; the racemate amlodipine was dissolved in n-decyl alcohol to make a concentration of 2.0g / L oil phase. Take 5 mL each of the water phase and the oil phase, put them together in a test tube, and shake them in a constant temperature shaker in a water bath for 5 hours, and control the extraction temperature to 25°C. After the extraction was completed, the test tube was left to stand for more than half an hour, and then the oil phase was taken out to detect the concentration of (R)-amlodipine and (S)-amlodipine. The concentrations of the two enantiomers in the aqueous phase were obtained by subtraction according to the law of conservation of mass. The distribution coefficient and enantioselectivity coeff...
Embodiment 2
[0026] A certain amount of 1-butyl-3-methylimidazolium glutamate is dissolved in the acetic acid-sodium acetate buffer solution (pH=5.5) with a concentration of 0.3mol / L to prepare an amino acid ionic liquid with a concentration of 0.025mol / L The aqueous phase of L; the amlodipine racemate is dissolved in n-decyl alcohol to make a concentration of 2.5g / L oil phase. Take 5 mL each of the oil phase and the water phase, put them together in a test tube, and extract at a constant temperature of 5 °C for 5 hours. Let the test tube stand still for more than half an hour, then take the oil phase to detect the concentration of the two enantiomers, and calculate the partition coefficient and enantioselectivity coefficient. The results showed that the partition coefficients of (R)-amlodipine and (S)-amlodipine were 11.98 and 15.45, respectively, and the enantioselectivity coefficient was 1.29.
[0027] Collect the extracted water phase, mix it with n-decyl alcohol in equal volume, then...
Embodiment 3
[0029]A certain amount of 1-butyl-3-methylimidazolium glutamate is dissolved in the acetic acid-sodium acetate buffer solution (pH=3.0) with a concentration of 0.6mol / L to make an amino acid ionic liquid with a final concentration of 0.3mol / L of the aqueous phase. Amlodipine racemate was dissolved in n-decyl alcohol to prepare an oil phase with a concentration of 5.0 g / L. Take 5 mL each of the oil phase and the water phase, put them together in a test tube, and extract at 15°C for 5 hours. After the two phases are completely separated, the test tube is left to stand for more than half an hour, then the oil phase is taken out to detect the enantiomer concentration of amlodipine, and the corresponding partition coefficient and enantiomer selectivity coefficient are calculated. The results showed that the enantioselectivity coefficient of amlodipine after extraction was 1.18, and the partition coefficients of (R)-amlodipine and (S)-amlodipine were 3.48 and 4.11, respectively. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com