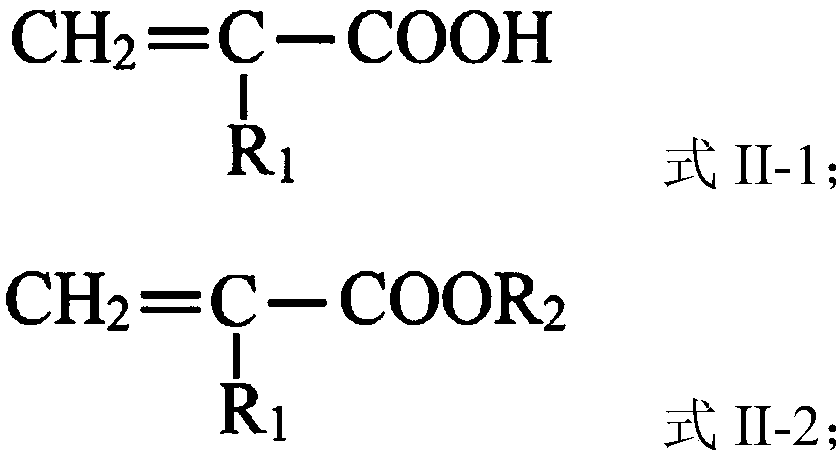Catalyst for preparing unsaturated acid or unsaturated acid ester
A technology of catalysts and compounds, which is applied in the direction of molecular sieve catalysts, physical/chemical process catalysts, and carboxylate preparations, etc. It can solve the problems of unsatisfactory large-scale production, easy loss of active components, and complex influencing factors, and achieves good industrial application prospects. , low cost, simple effect of industrial preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 catalyst
[0059] Na-Molecular sieves with FER configuration
[0060] Na-FER molecular sieves were purchased from Shanghai Zhuoyue Chemical Technology Co., Ltd.
[0061] Table 1 Na-FER molecular sieves with three different average particle sizes
[0062] Na-FER
Si / Al
Grain size (μm)
D. 50 (μm)
Sample A
2 / 6.5
0.1-0.5
1.6
Sample B
30 / 7
0.5-1.5
3.0
Sample C
50 / 6.5
1.5-3.5
4.8
[0063] Preparation of H-FER
[0064] Na-FER catalyst was purchased from Shanghai Zhuoyue Chemical Technology Co., Ltd. 10 grams of calcined Na-molecular sieves with FER configuration were exchanged three times with 0.5mol / L ammonium nitrate for 2 hours each time, washed with deionized water, dried, calcined at 550°C for 4 hours, and prepared by extrusion into 20 ~40 mesh H-FER catalyst, samples A, B, and C were prepared as catalyst samples 1#, 2#, and 3#, respectively.
[0065] Preparatio...
Embodiment 2
[0091] Embodiment 2 methylal DMM reacts with methyl acetate MAc
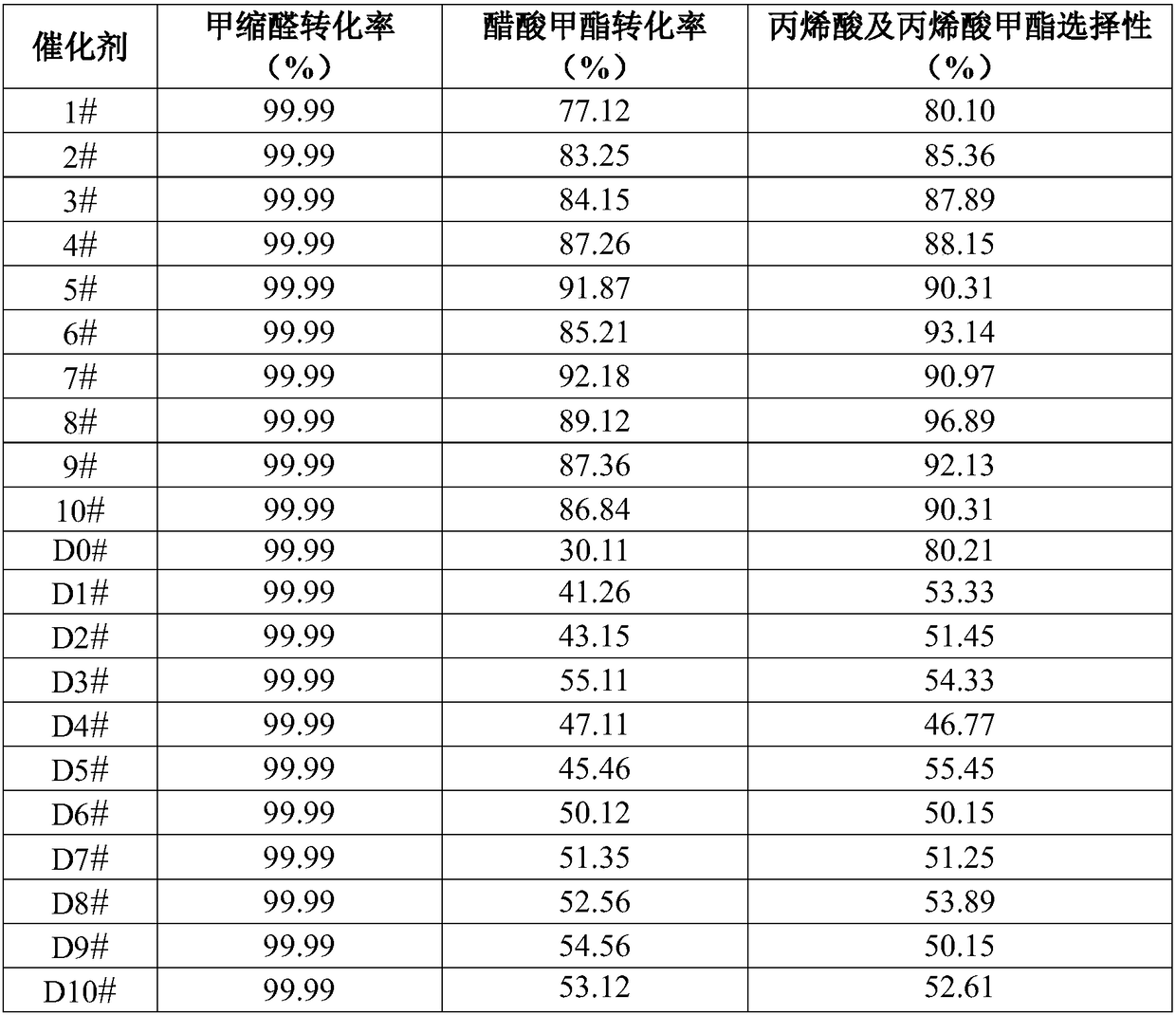
[0092]10g of the above-mentioned catalyst samples 1# to 10# and catalyst samples D0# to D10# were respectively loaded into a fixed-bed reactor with a tube inner diameter of 28mm, and the temperature was raised to 550°C at 5°C / min under a nitrogen atmosphere and kept for 4 hours. Then the nitrogen atmosphere was lowered to the reaction temperature of 350° C., and the pressure of the reaction system was raised to the reaction pressure of 3 MPa with nitrogen. The reaction raw materials (wherein the molar ratio of methylal and methyl acetate is 2:1) from top to bottom at a total feed space velocity of 0.3h -1 Through the catalyst bed, the catalyst evaluation results are shown in Table 3. After 30 hours of reaction time, samples were taken and analyzed, and the results are shown in Table 3.
[0093] Table 3 Catalyst evaluation results for the reaction between methylal DMM and methyl acetate MAc
[0094]
Embodiment 3
[0095] Embodiment 3 different reaction conditions
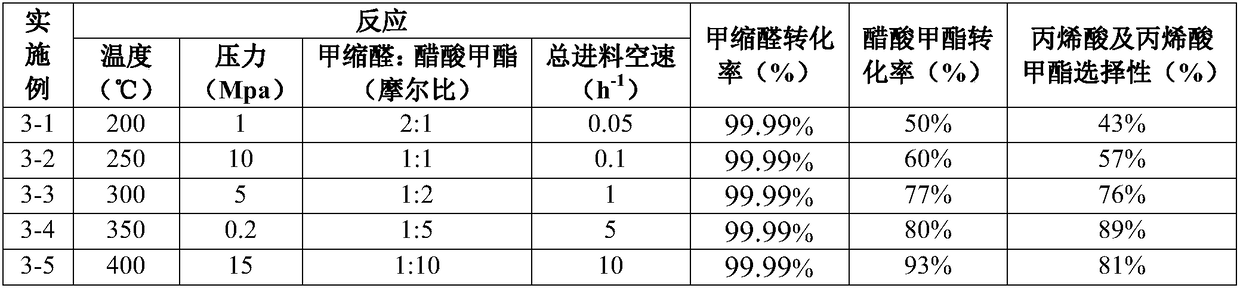
[0096] Put 10g of the above-mentioned 10# catalysts into the column tubes respectively, pass through a 28mm fixed bed reactor, raise the temperature to 550°C at 5°C / min under nitrogen atmosphere, and keep it for 4 hours. Then the nitrogen atmosphere is lowered to a reaction temperature of 200°C to 400°C, the DMM / MAc molar ratio is 2 / 1, and the pressure of the reaction system is raised to a reaction pressure of 0.2Mpa to 15.0Mpa with nitrogen. Feed the raw materials from top to bottom at a total feed space velocity of 0.05h -1 to 10.0h -1 through the catalyst bed. The specific reaction conditions are shown in Table 4. After 30 hours of reaction time, samples were taken for analysis, and the results are shown in Table 4.
[0097] Pretreatment and reaction conditions of the catalyst of table 4
[0098]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap