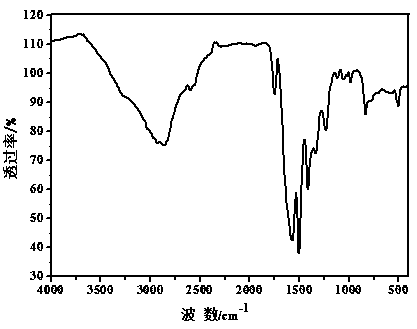
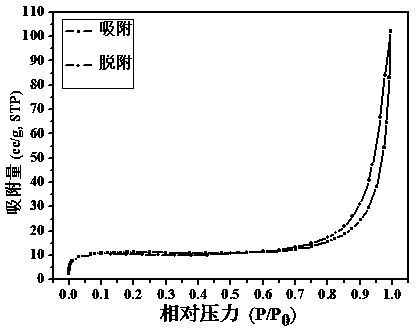
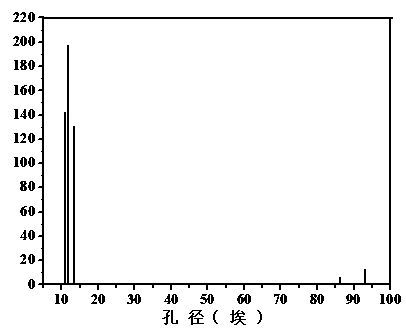
Synthesis method of porous organic covalent frame material with triazine structure
A frame material and synthesis method technology, which is applied in the field of synthesis of organic porous frame materials, can solve the problems of high cost, complicated experimental process, and high technical requirements, and achieve the effects of low synthesis cost, simple synthesis process, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve 0.9733g of p-phenylenediamine and 1.107g of cyanuric chloride (the molar ratio of substances is 3:2) in 35 ml and 11ml of dioxane-mesitylene-acetic acid mixed solvents respectively (the volume ratio of the three solvents 5:5:1), then mix the two reactant solutions evenly, transfer to a stainless steel autoclave, keep at 120°C for 70 hours, and wash the product repeatedly with tetrahydrofuran and methanol. After washing, vacuum dry at 120°C for 24 hours , the khaki powder obtained after grinding is the target product. The yield is about 82%, and the specific surface area of the product is 33.6447 m 2 ∙g -1 , with a pore size of about 1.37 nm.
Embodiment 2
[0033] Dissolve 0.9733g p-phenylenediamine and (0.5535g) cyanuric chloride (ratio of substance is 3:1) in 35ml and 10ml dioxane-mesitylene-acetic acid mixed solvent respectively (three solvents The volume ratio is 4.5:4.5:1), and then the two reactant solutions are mixed evenly, transferred to a stainless steel high-pressure reactor, and kept at 120°C for 70 hours. The product is washed repeatedly with tetrahydrofuran and methanol. After washing, it is vacuum-dried at 120°C After 24 hours, the yellow powder obtained after grinding was the target product. The yield is about 80%, and the specific surface area of the product is 15m 2 ∙g -1 , with a pore size of about 1.5 nm.
[0034] Compared with Example 1, when other conditions are constant, increasing the ratio of p-phenylenediamine and cyanuric chloride will make the specific surface area of the product smaller, because unreacted p-phenylenediamine is more difficult to It is separated from the reaction product and atta...
Embodiment 3
[0036] Dissolve 0.9733g p-phenylenediamine and 1.107g cyanuric chloride (mass ratio is 3:2) in 35ml and 10ml dioxane-mesitylene-acetic acid mixed solvent respectively (three kinds of solvent volume ratio 4:4:1), then mix the two reactant solutions evenly, transfer them to a stainless steel autoclave, keep at 120°C for 70 hours, and wash the product repeatedly with tetrahydrofuran and methanol. After washing, dry it under vacuum at 120°C for 24 hours , the khaki powder obtained after grinding is the target product. The yield is about 87%, and the specific surface area of the product is 9 m 2 ∙g -1 , with a pore size of about 1.8 nm.
[0037] Compared with Example 1, under the condition that other conditions are constant, the ratio of three kinds of solvents is adjusted, and it is found that the solubility of the reactant decreases, and the specific surface area of the product decreases. It is not conducive to the production of qualified products.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com