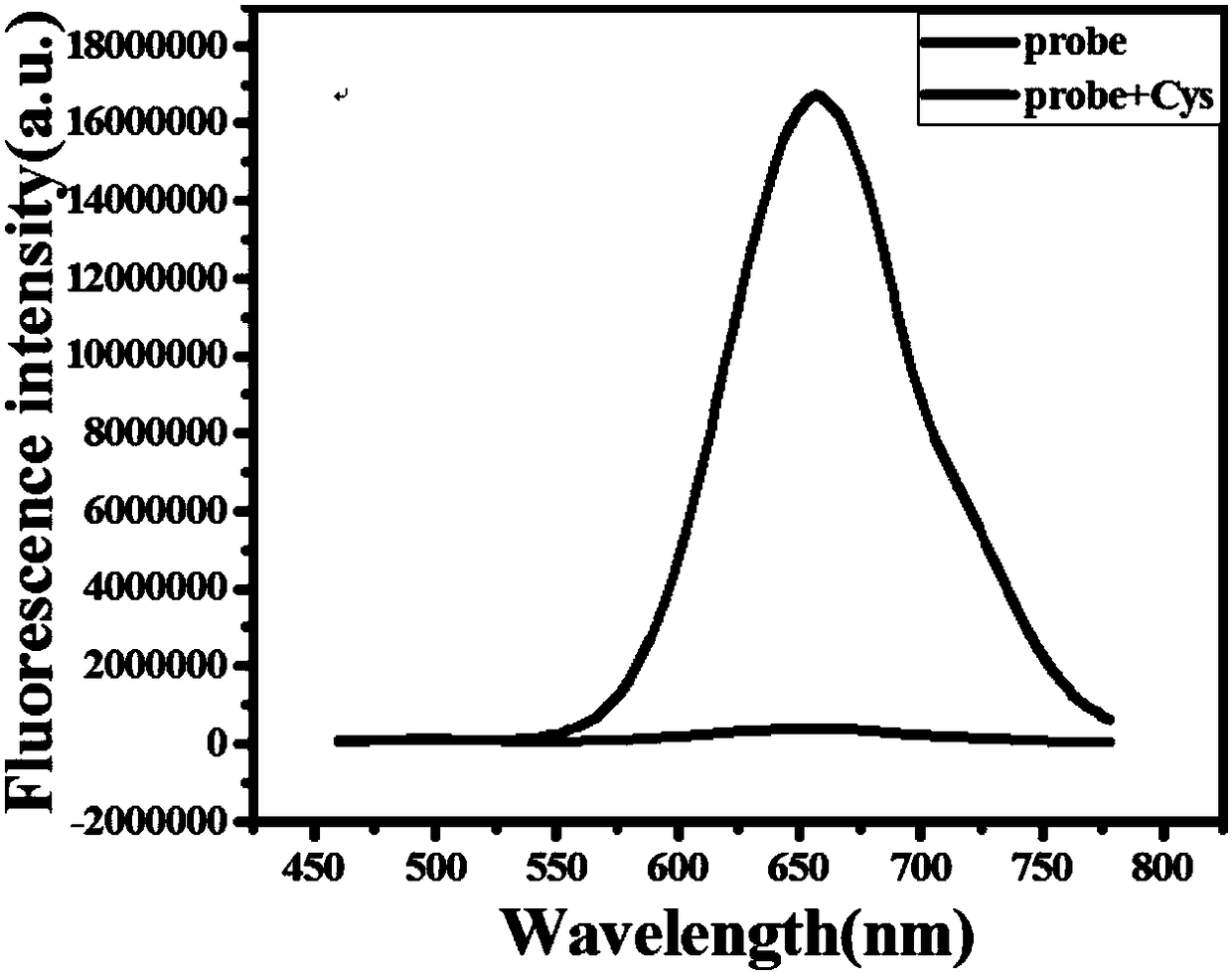
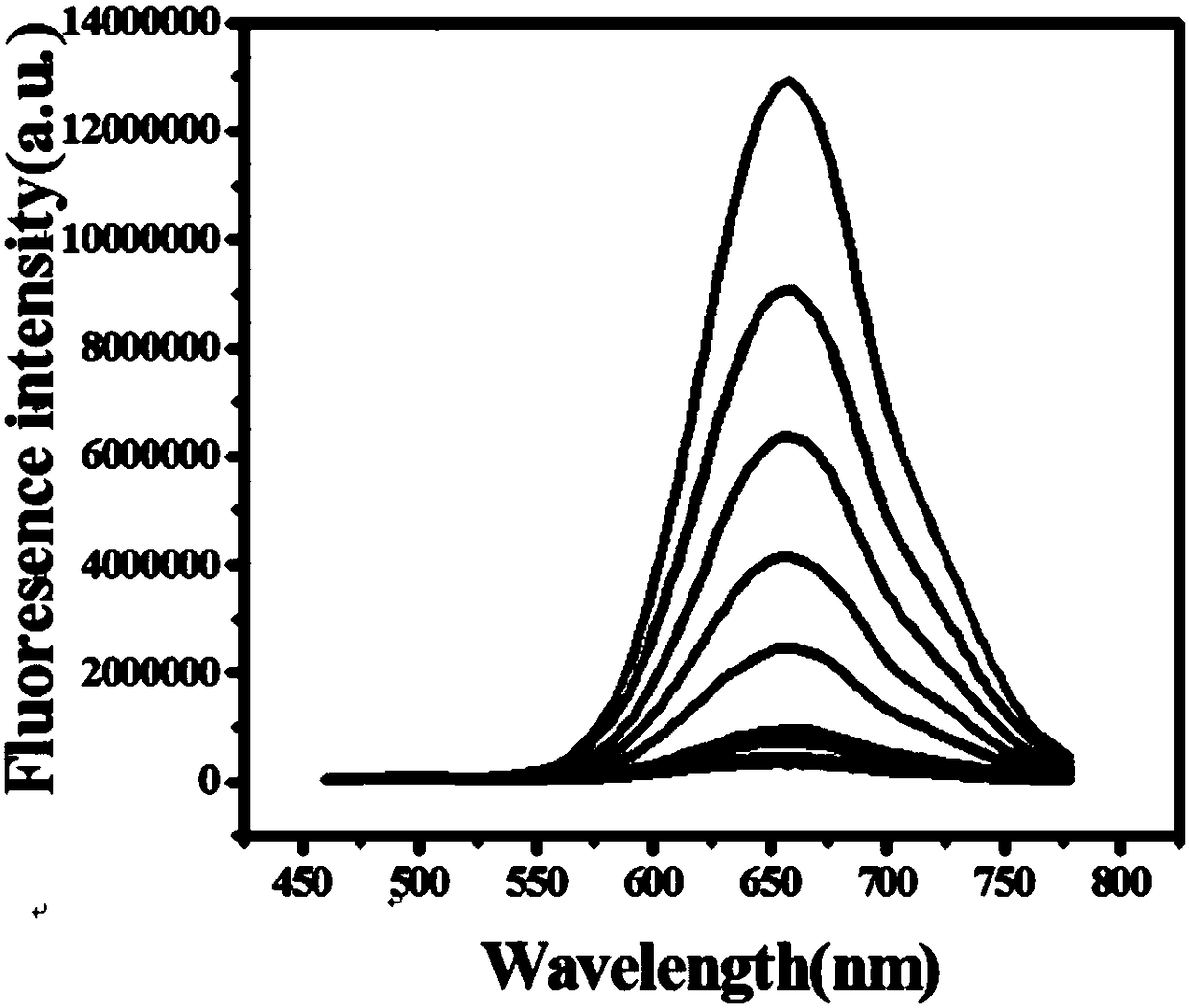
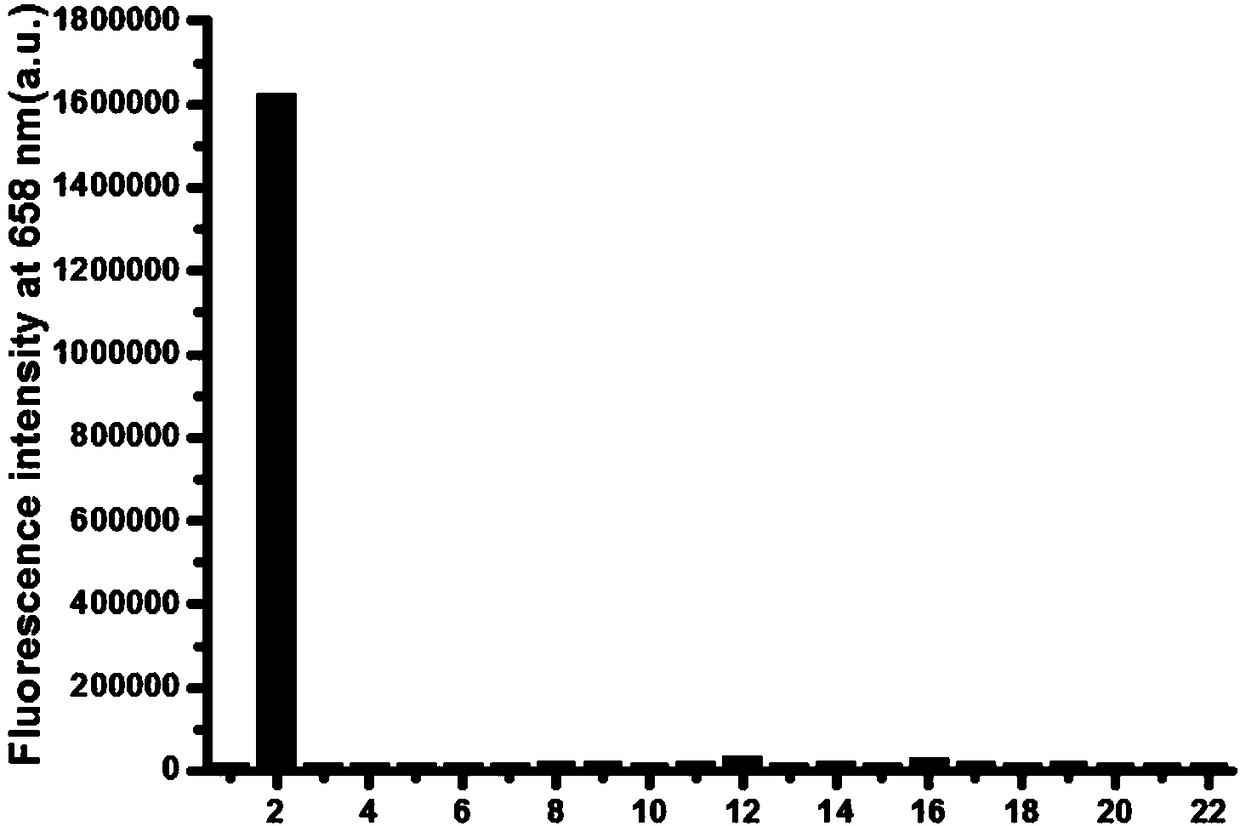
Fluorescent probe for near infrared detection of cysteine as well as preparation method and application of fluorescent probe
A near-infrared detection and fluorescent probe technology, applied in the field of chemical analysis and detection, can solve the problems of biological imaging application limitations, inability to effectively distinguish cysteine and homocysteine, etc., and achieve high sensitivity and sample penetration Strong, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A preparation method of a fluorescent probe, the preparation method comprising the following steps:
[0043]
[0044] The specific experimental operation is as follows:
[0045] Synthesis of 4-piperazine-1-benzaldehyde:
[0046] Add 15g (174.14mmol) of piperazine to a 100mL two-necked round-bottom flask, then add 18mL of water and 25mL of ethylene glycol methyl ether; measure 5mL (46.61mmol) of p-fluorobenzaldehyde with a graduated cylinder, and then add it to a constant pressure dropping funnel , add 5mL of ethylene glycol methyl ether to mix with it, and slowly drop it into the above-mentioned reaction flask under stirring; after the dropwise addition at room temperature, heat and reflux for 4 hours; wait for the reaction solution to cool to room temperature, pour the reaction solution into 200mL of water, and filter. Add the filter cake to 150mL of 10% hydrochloric acid solution and stir for 10 minutes, filter to remove insoluble matter, adjust the pH of the solu...
Embodiment 2
[0052] The processes of this embodiment and Embodiment 1 are basically the same, where the differences are:
[0053] The molar ratio of piperazine to p-fluorobenzaldehyde is 3:1 (139.83mmol:46.61mmol);
[0054] The molar ratio of 4-piperazine-1-benzaldehyde and 2-(3,5,5-trimethylcyclohex-2-enylidene) malononitrile is 1:1.05 (2.63mmol:2.76mmol);
[0055] The molar ratio of 2-(3,5,5-trimethylcyclohex-2-enylidene)malononitrile to 2,4-dinitrobenzenesulfonyl chloride was 1:1.05 (1 mmol:1.05 mmol).
[0056] In this example, the final result is the same as the experimental product in Example 1, indicating that the fluorescent probe can also be successfully synthesized under the above-mentioned ratio.
Embodiment 3
[0058] The processes of this embodiment and the embodiment are basically the same, where the differences are:
[0059] The molar ratio of piperazine to p-fluorobenzaldehyde is 4:1 (186.44mmol: 46.61mmol);
[0060] The molar ratio of 4-piperazine-1-benzaldehyde and 2-(3,5,5-trimethylcyclohex-2-enylidene) malononitrile is 1:1 (2.63mmol: 2.63mmol);
[0061] The molar ratio of 2-(3,5,5-trimethylcyclohex-2-enylidene)malononitrile and 2,4-dinitrobenzenesulfonyl chloride was 1:1.05 (1 mmol: 1 mmol).
[0062] In this example, the final result is the same as the experimental product in Example 1, indicating that the fluorescent probe can also be successfully synthesized under the above-mentioned ratio.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com