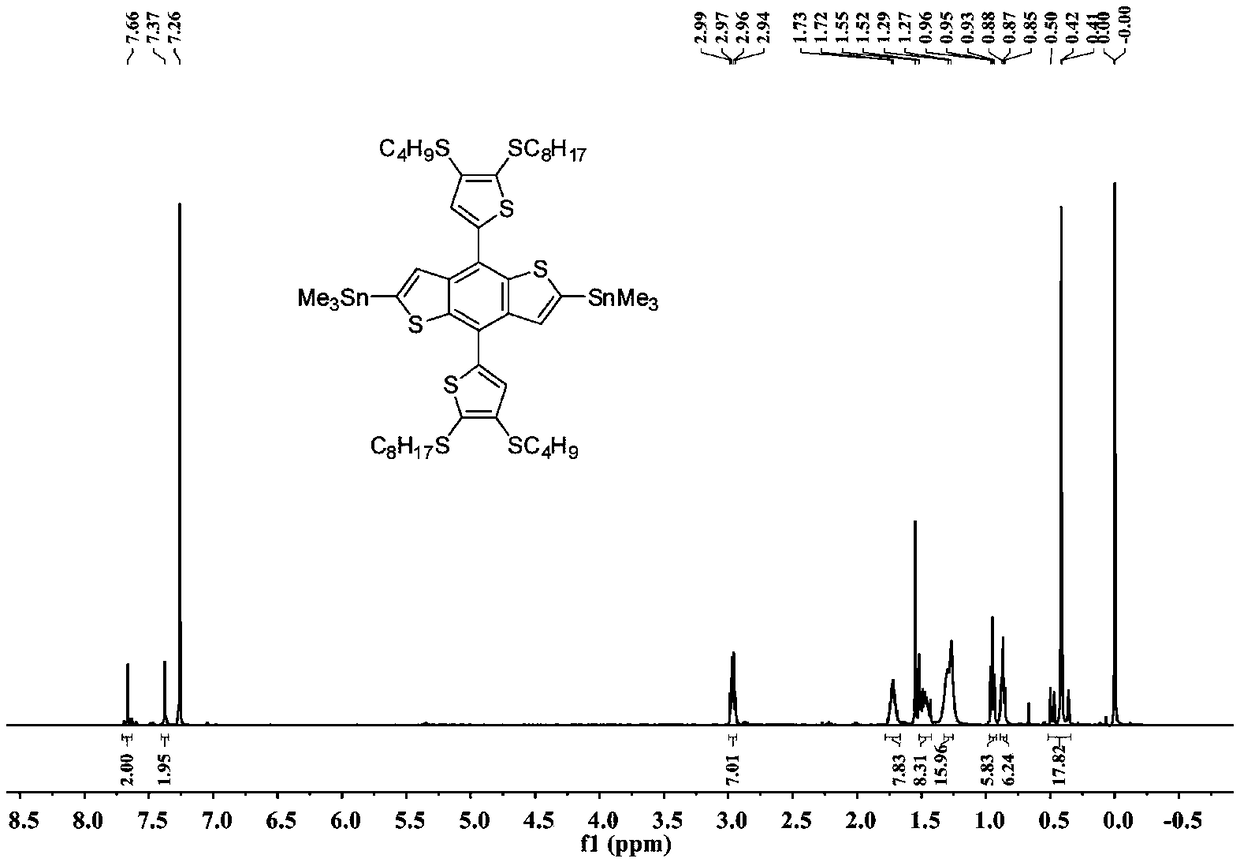
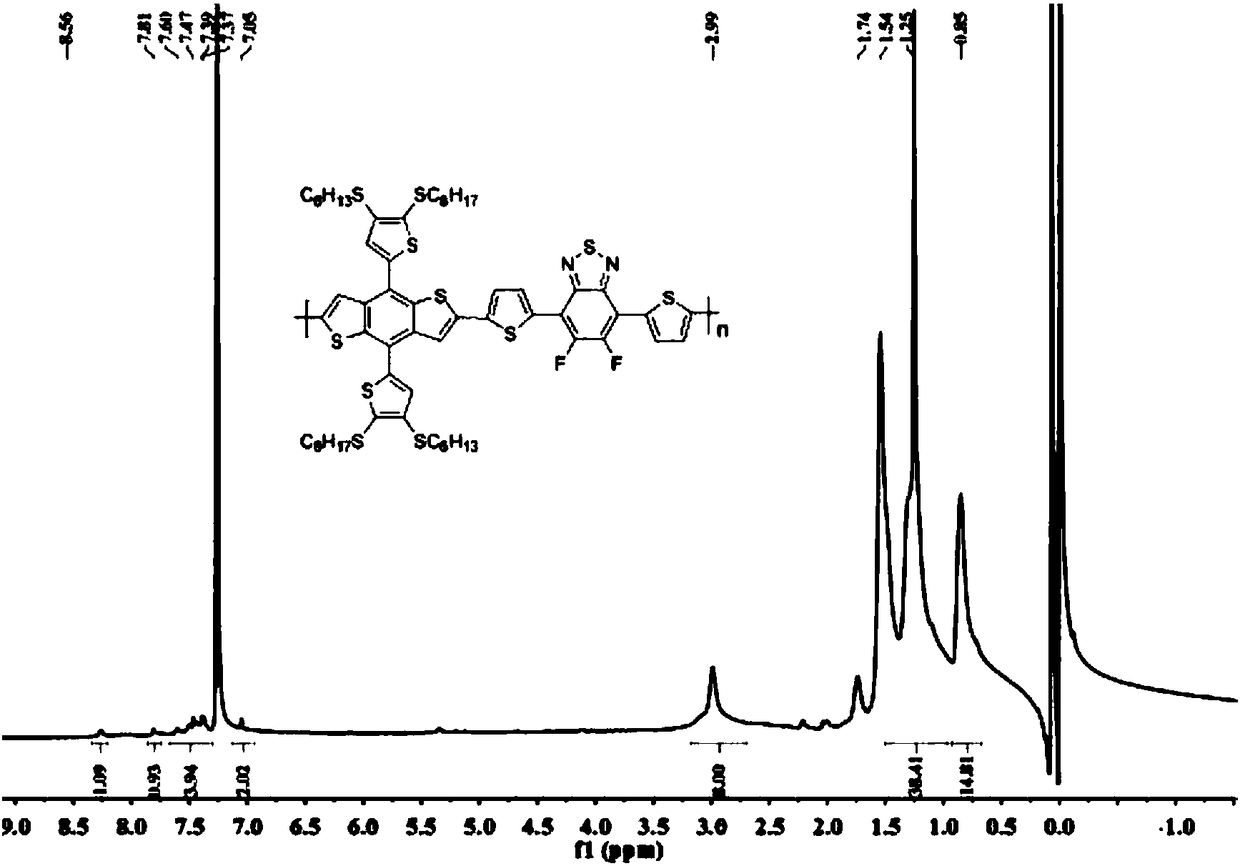
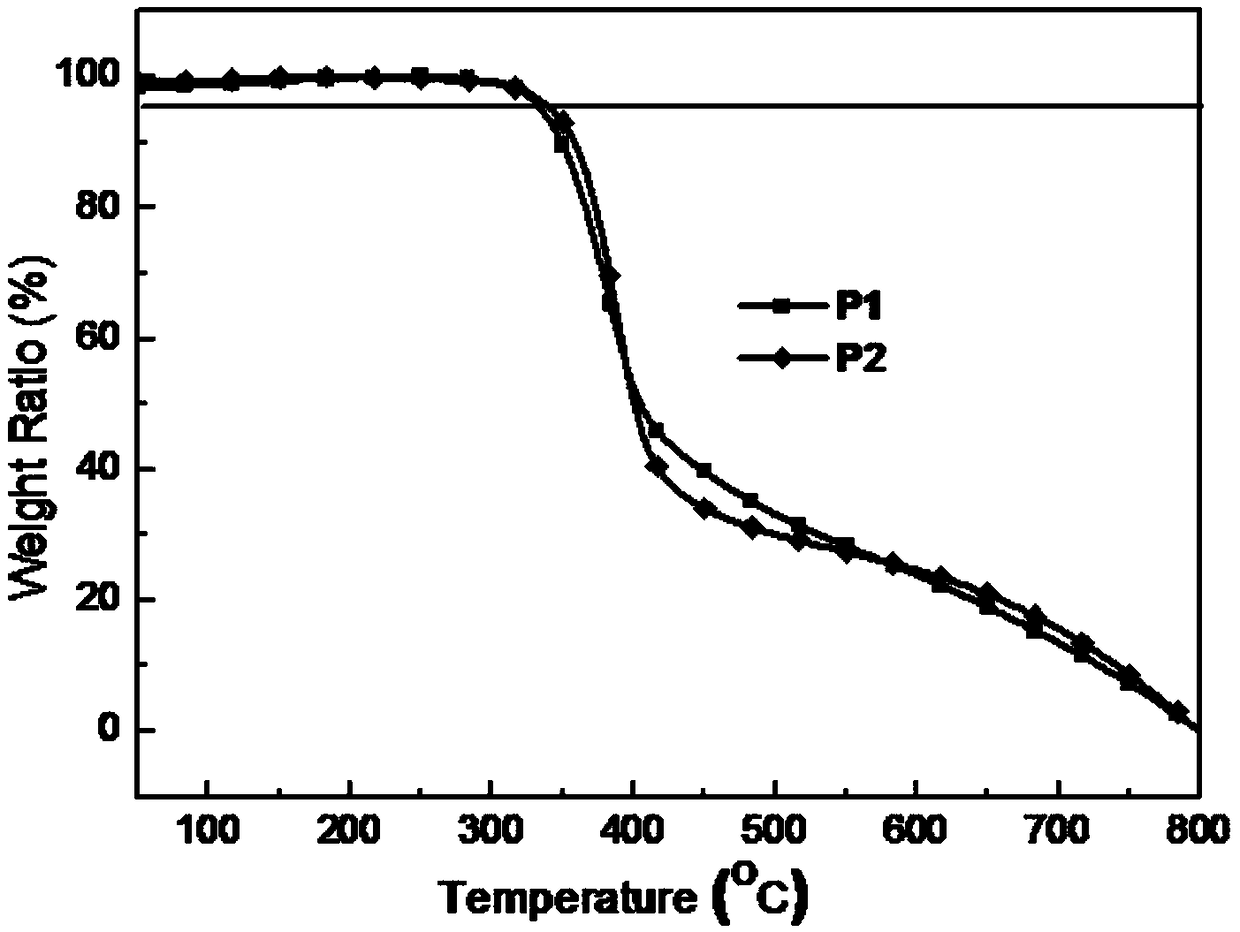
Two-dimensional conjugated polymer electron donor material containing disulfhydryl substituted side chain
A conjugated polymer and electron technology is applied in the field of two-dimensional benzodithiophene conjugated polymer electron donor materials and their preparation, and achieves the effects of good electrochemical properties and wide spectral absorption range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the D-A type conjugated polymer electron donor material in the polymer solar cell blending active layer of the present invention comprises the following steps:
[0034] a. Add 3-carboxythiophene and dehydrated dichloromethane into an anhydrous two-necked flask, add oxalyl chloride dropwise at 0° C., and stir overnight. The solvent and excess oxalyl chloride were removed by a rotary evaporator, and 1 was obtained as a colorless solid after drying.
[0035] b. Dissolve compound 1 in dehydrated dichloromethane under the protection of nitrogen, add dropwise a solution of diethylamine dissolved in dichloromethane at 0° C., and stir overnight. After the reaction was completed, it was poured into deionized water for extraction, and the organic phases were combined and washed with saturated brine, dried over anhydrous magnesium sulfate and filtered. After removing the solvent, the crude product was separated by column chromatography to obtain yellow li...
Embodiment 1
[0046] (1) 3-Butylthiothiophene
[0047]
[0048] The raw material 3-bromothiophene (6.0g, 36.80mmol), butanethiol (4.35ml, 40.48mmol) was added to anhydrous toluene, and 1,1'-bis(diphenylphosphino)ferrocene ( 816.1mg, 1.47mmol), N,N-diisopropylethylamine (5.23g, 40.48mmol) and Pd 2 (dba) 3 (337.0 mg, 0.37 mmol), then heated to 110 ° C for 12 hours; stopped the reaction, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, spin-dried the organic solvent, and separated by column chromatography to obtain a yellow liquid with a yield of 95 %. Among them, 3-bromothiophene and butanethiol, 1,1’-bis(diphenylphosphino)ferrocene, N,N-diisopropylethylamine, Pd 2 (dba) 3 The molar ratio is 1:1.1:0.04:1.1:0.02.
[0049] (2) 2-Bromo-3-butylthiothiophene
[0050]
[0051] 3-Butylthiothiophene (5.8g, 33.66mmol) was dissolved in dimethylformamide, and after cooling down to 0°C, N-bromosuccinimide (6.0g, 33.66 mmol), reacted at 0°C for 4h, extracted with ethyl ac...
Embodiment 2
[0067] (1) 3-Hexylthiothiophene
[0068]
[0069] Raw materials 3-bromothiophene (6.0g, 36.80mmol), hexane mercaptan (4.79g, 40.48mmol) were added to anhydrous toluene, and N,N-diisopropylethylamine (5.23g, 40.48mmol ), 1,1'-bis(diphenylphosphino)ferrocene (816.1mg, 1.47mmol) and Pd 2 (dba) 3 (337.0mg, 0.37mmol), heated to 110°C for 12 hours; stopped the reaction, extracted with ethyl acetate, and separated by column chromatography to obtain a yellow liquid with a yield of 93%. Among them, 3-bromothiophene and butanethiol, N,N-diisopropylethylamine, 1,1’-bis(diphenylphosphino)ferrocene, Pd 2 (dba) 3 The molar ratio is 1:1.1:1.1:0.04:0.02.
[0070] (2) 2-bromo-3-hexylthiothiophene
[0071]
[0072] Dissolve 3-hexylthiothiophene (5.3g, 26.45mmol) in dimethylformamide, lower the temperature to 0°C under nitrogen protection and stir for 30 minutes, then add N-bromosuccinimide (4.71 g, 26.45mmol), reacted at 0°C for 4h, extracted the product with ethyl acetate after the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com