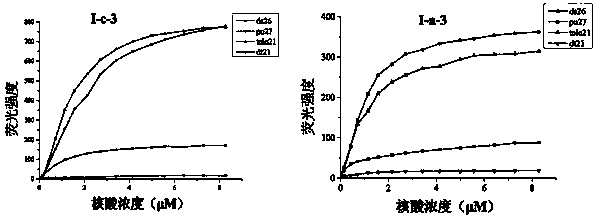
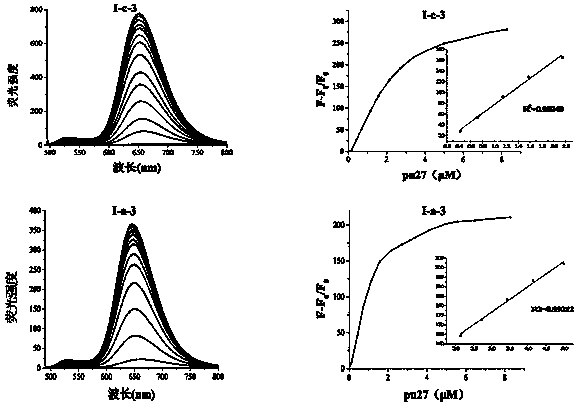
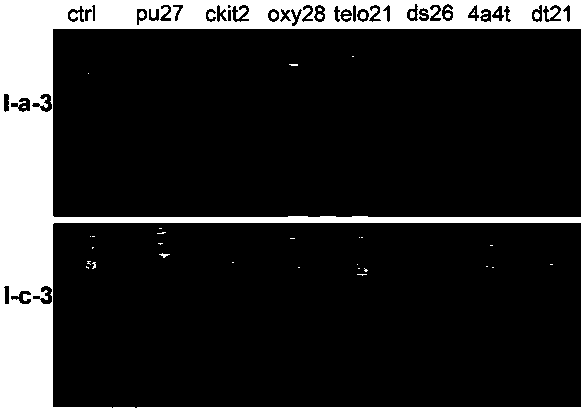
Nucleic acid dye compound and preparation method and application of nucleic acid dye compound
A nucleic acid dye and compound technology, applied in the field of fluorescent probe probes, can solve the problems of slow response speed of G-quadruplex DNA or RNA, affecting application value, high phototoxicity, etc. Biotoxic, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The present invention also provides a preparation method of nucleic acid dye compound, comprising:
[0052] The compound of formula (II) structure is reacted with the compound of formula (III) structure to obtain the compound of formula (I) structure,
[0053] R 3 -CHO formula (III),
[0054] Among them, R 1 Alkylamino, piperidinyl, morpholinyl, pyrrolidinyl, piperazinyl or pyroxolinyl selected from C1-C6;
[0055] R 2 Aryl vinyl selected from C8-C20;
[0056] R 3 It is selected from a C8-C20 aryl group or a C8-C20 aryl vinyl group.
[0057] According to the present invention, according to the present invention, the present invention will react the compound of formula (II) structure with the compound of formula (III) structure, obtain the compound of formula (I) structure, wherein, the present invention does not have special requirement to the condition of reaction , those skilled in the art can select suitable reaction conditions according to common knowledg...
Embodiment 1
[0079] Embodiment 1: the synthesis of compound 1a
[0080] Weigh 0.2g (1.130mmol) of 4-chlorodimethylquinoline into a 25ml round bottom flask, add about 1.2g of methyl iodide and 5.0ml of sulfolane, and heat the mixture to 60°C for 6 hours of reaction After cooling, shake after adding ethyl acetate, suction filter, wash the solid with ethyl acetate, weigh after vacuum drying, thin-layer chromatography preliminary shows that there is no by-product, obtains 1.82g pure product 1a, chemical structural formula is as follows, yield was 88.6%.
[0081]
[0082] The compound 1a obtained in Example 1 is analyzed by nuclear magnetic resonance, and its hydrogen nuclear magnetic resonance spectrum result is obtained: 1H NMR (400MHz, DMSO) δ8.67 (d, J=9.0Hz, 1H), 8.58-8.50 (m, 2H), 8.33 (ddd, J = 8.7, 7.1, 1.3 Hz, 1H), 8.11 (dd, J = 13.4, 5.7 Hz, 1H), 4.43 (s, 3H), 3.07 (s, 3H).
[0083] Compound 1a obtained in Example 1 is analyzed by mass spectrometer, and its mass spectrometry resu...
Embodiment 2
[0084] Embodiment 2: the synthesis of compound 1b
[0085] Weigh 0.4g (1.56mmol) of 2-methylbenzothiazole and 0.4ml (2mmol) of 1,3-dibromopropane into a 25ml round-bottomed flask as a pinkish white solid, heat the mixture to 100°C, and react 12 After one hour, cool, shake after adding ethyl acetate, suction filter, solid is washed with ethyl acetate, weigh after vacuum drying, thin-layer chromatography preliminary shows that there is no by-product, chemical structural formula is as follows, pure product 1b, productive rate is 98.2%.
[0086]
[0087] Compound 1b obtained in Example 2 was analyzed by nuclear magnetic resonance, and its hydrogen nuclear magnetic resonance spectrum result was obtained: 1H NMR (400MHz, DMSO) δ8.67 (d, J=9.0Hz, 1H), 8.57-8.51 (m, 2H), 8.33 (ddd, J = 8.7, 7.1, 1.3 Hz, 1H), 8.12 (t, J = 7.7 Hz, 1H), 4.43 (s, 3H), 3.07 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com