Method for modifying lemon exosome
A modification method, exosome technology, applied in the field of DNA recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 (preparation of PEG-cRGD-lemon exosomes)
[0019] 1. Extract lemon exosomes
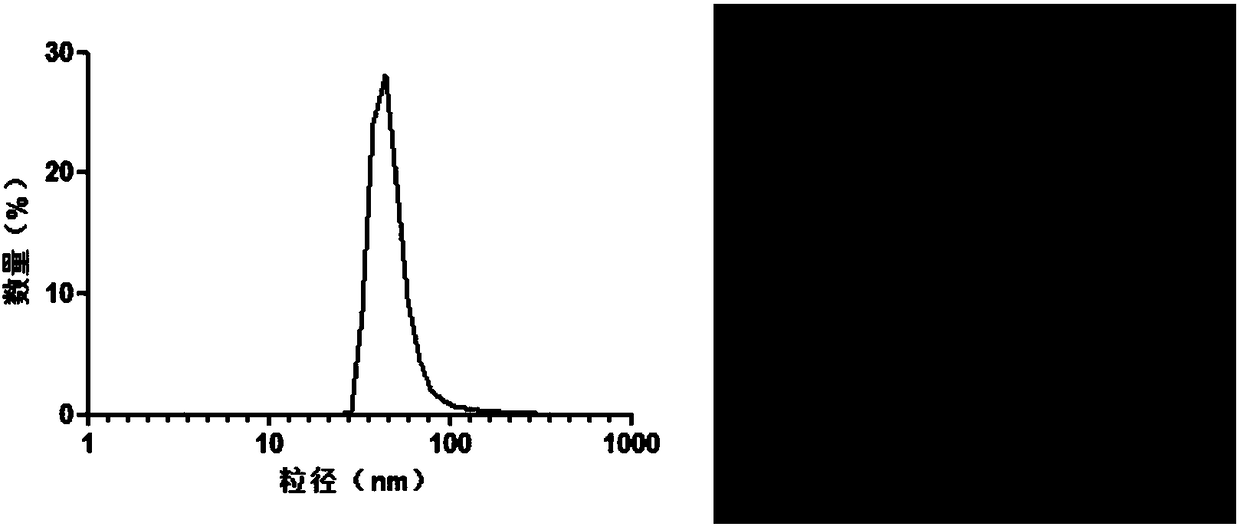
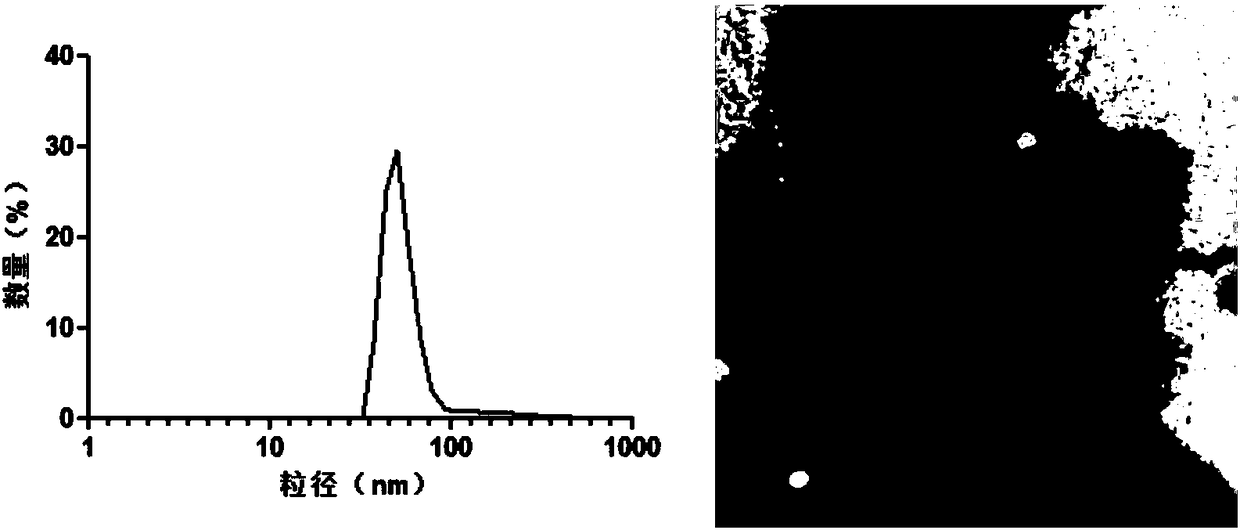
[0020] Purchase fresh lemons, peel and squeeze juice, dilute the juice with PBS buffer, and centrifuge at a differential speed (centrifuge at 500g for 10min, centrifuge at 2000g for 20min, centrifuge at 5000g for 30min, centrifuge at 10000g for 1h to get the supernatant, and centrifuge the supernatant at 100000g for 2h to get the precipitate) to obtain the outer Exosome precipitation, PBS buffer resuspension, sucrose density gradient centrifugation (8%, 30%, 45%, 60% sucrose were dissolved in 20mM Tris.Cl respectively) to purify exosomes, dynamic light scattering and transmission electron microscopy Identify the particle size distribution and morphology characterization of exosomes, such as figure 1 shown. The BCA protein quantification method was used to measure the protein content in exosomes, and the obtained exosomes were stored at -80°C for later use.
[0021] 2. (Modified le...
Embodiment 2
[0027] Embodiment 2 (drug effect test)
[0028] (1) The effect on the growth of glioma cells
[0029] In the logarithmic growth phase of the cells, the cell suspensions of human glioma cells U87 and U251 were inoculated in 96-well plates, with about 3500 cells per well, and the 96-well plates were placed at 37°C and 5% CO 2 Cultivate in the incubator for 12 hours, set up 5 exosome concentration gradients for dosing, the highest concentration is 20 μg / ml, and serially dilute until the concentration is 0, and set 5 duplicate wells for each concentration. After adding the medicine, the 96-well plate Placed at 37°C, 5% CO 2 Cultivate for 48h, then add MTT to continue to cultivate for 4h, microplate reader detects the absorbance value (OD value) (detection wavelength 490nm), calculates the relative survival rate of cells under each drug concentration respectively, the result is as follows image 3 shown. Relative cell viability (%)=(OD 实验组 mean / OD 对照组 mean) x 100%.
[0030] Dep...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap