Bicyclic derivatives of glucoside and its preparation method and use
A technology of diols and compounds, applied in the field of medicine and chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] The preparation of embodiment 1,5-bromo-2-chloro-4'-ethoxybenzophenone (Ⅴ-1)
[0177]
[0178] Add 5-bromo-2-chlorobenzoic acid (20.0g, 0.085mol) to 2.0M oxalyl chloride in dichloromethane solution (50ml, 0.1mol), stir to form a suspension, then add 8 drops of DMF solution dropwise, there are bubbles After 3 hours of reaction, the reaction is basically complete, and the solvent is spin-dried on a rotary evaporator, then 15ml of dichloromethane is added, and the solvent is spin-dried. After spin-drying, add 30ml of dichloromethane, stir, cool down to 0-5°C, add phenetole (10.9g, 0.089mol), add anhydrous aluminum chloride (12.5g, 0.093mol) in batches, control the temperature If the temperature is higher than 5°C, continue to stir at 4°C for 1 hour after the addition is complete. TLC monitors that the reaction is almost complete. Place the reactant on an ice-water mixture to quench the reaction, separate the organic phase, extract the aqueous phase with dichloromethane...
Embodiment 2
[0181] Preparation of embodiment 2, 5-bromo-2-chloro-4'-ethoxydiphenylmethane (Ⅲ-1)
[0182]
[0183] Add 5-bromo-2-chloro-4'-ethoxybenzophenone (V-1) (18.0 g, 0.053 mol) in 135 ml of acetonitrile / dichloromethane mixed solvent (volume ratio 2:1), Cool down to 5-10°C, add triethylsilane (18.6ml, 0.117mol), then add boron trifluoride diethyl ether (9.4ml, 0.074mol) dropwise, remove the ice bath after the dropwise addition, and let it rise to room temperature to react overnight . TLC monitors that the reaction is almost complete, adding saturated sodium bicarbonate solution to adjust the pH to neutral, extracting with ethyl acetate, washing the organic phase twice with saturated sodium chloride, and drying over anhydrous sodium sulfate. Filter with suction and spin dry the solvent to obtain an oily substance. 13.2 g (76.5%) of oil were obtained after column chromatography.
[0184] 1 H-NMR (400MHz, CDCl 3 , ppm- 1 )δ: 7.23(m,3H), 7.08(m,2H), 6.83(m,2H), 4.01(q,2H), 3.9...
Embodiment 3
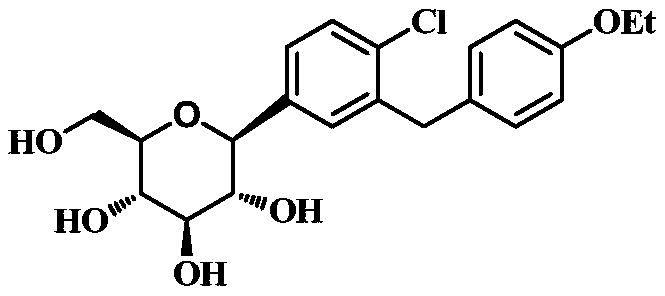
[0185] Example 3, methyl 1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-α-D-glucopyranoside (Ⅱ-1) Preparation
[0186]
[0187] Under nitrogen protection, add 5-bromo-2-chloro-4'-ethoxydiphenylmethane (Ⅲ-1) (12.5, 0.038mol), 40ml dry tetrahydrofuran and 80ml toluene into a 250ml three-necked flask (numbered bottle A) (dry with calcium chloride), cool down to -72°C with dry ice acetone under stirring, add 2.5M n-BuLi n-hexane solution (18.4ml, 0.046mol) dropwise, make sure the temperature is not higher than -60°C, and stir for 30min. Prepare another 500ml three-necked bottle (number B), nitrogen protection, add 2,3,4,6-tetra-O-trimethylsilyl-β-D-gluconolactone (19.7g, 0.042mol) and 110ml of toluene (dried over calcium chloride), cooled to -72°C with dry ice acetone. When the reaction time in bottle A is up, introduce the solution in bottle A into bottle B, continue the reaction at low temperature for 2 hours, add methanesulfonic acid (14.8g, 0.154mol) and 110ml methano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com