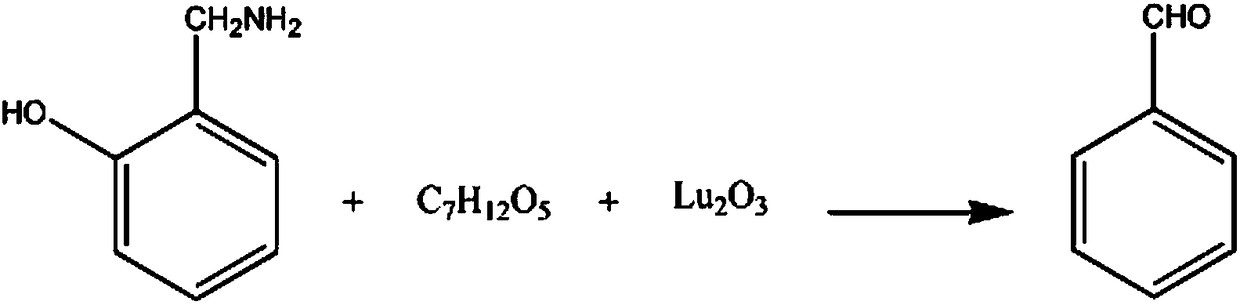Mandelic acid drug intermediate benzaldehyde synthesis method
A synthesis method and technology of benzaldehyde, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve the problems of high reaction energy consumption, complex synthesis process, high risk factor, etc., and achieve an increase in reaction yield , Improve the reaction yield and reduce the risk factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthetic method of mandelic acid medicine intermediate benzaldehyde comprises the steps:
[0019] A. Add 2mol of 1-aminomethyl-6-hydroxybenzene and 1.3L of potassium chloride solution with a mass fraction of 20% in the reaction vessel, control the stirring speed to 210rpm, increase the temperature to 60°C, keep reflux for 80min, and reduce When the temperature reaches 30°C, add 3 mol of a 40% glycerol diacetate solution;
[0020] B. Add 2 mol of lutetium oxide in 3 times within 30 minutes, continue to react for 60 minutes, add 10% potassium nitrate solution in mass fraction, let stand for stratification, take out oil layer, wash with 50% amyl formate solution with mass fraction, Wash with 60% chloroacetonitrile solution, recrystallize in 80% 2-chloroethanol solution, and dehydrate with anhydrous sodium sulfate dehydrating agent to obtain 186.55 g of finished benzaldehyde with a yield of 88%.
Embodiment 2
[0022] The synthetic method of mandelic acid medicine intermediate benzaldehyde comprises the steps:
[0023] A. Add 2mol of 1-aminomethyl-6-hydroxybenzene into the reaction vessel, 1.3L of 23% potassium chloride solution by mass fraction, control the stirring speed to 230rpm, raise the temperature to 63°C, keep reflux for 90min, and lower the temperature To 33°C, add 3.5 mol of 42% glycerol diacetate solution;
[0024] B. Add 2.5mol of lutetium oxide in 5 times within 40min, continue to react for 70min, add mass fraction and be 12% potassium nitrate solution, let stand for stratification, take out the oil layer, wash with 53% amyl formate solution with mass fraction, Wash with 63% chloroacetonitrile solution, recrystallize in 83% 2-chloroethanol solution, and dehydrate with anhydrous calcium sulfate dehydrating agent to obtain 195.04 g of finished benzaldehyde with a yield of 92%.
Embodiment 3
[0026] The synthetic method of mandelic acid medicine intermediate benzaldehyde comprises the steps:
[0027] A. Add 2mol of 1-aminomethyl-6-hydroxybenzene into the reaction vessel, 1.3L of 26% potassium chloride solution by mass fraction, control the stirring speed to 250rpm, raise the temperature to 68°C, keep reflux for 110min, lower the temperature To 37°C, add 4 mol mass fraction of 45% glycerol diacetate solution;
[0028] B. Add 3 mol of lutetium oxide in 7 times within 50 minutes, continue to react for 80 minutes, add 15% potassium nitrate solution in mass fraction, let stand for stratification, take out oil layer, wash with 57% amyl formate solution in mass fraction, Wash with 66% chloroacetonitrile solution, recrystallize in 87% 2-chloroethanol solution, and dehydrate with anhydrous potassium carbonate dehydrating agent to obtain 199.28 g of finished benzaldehyde with a yield of 94%.
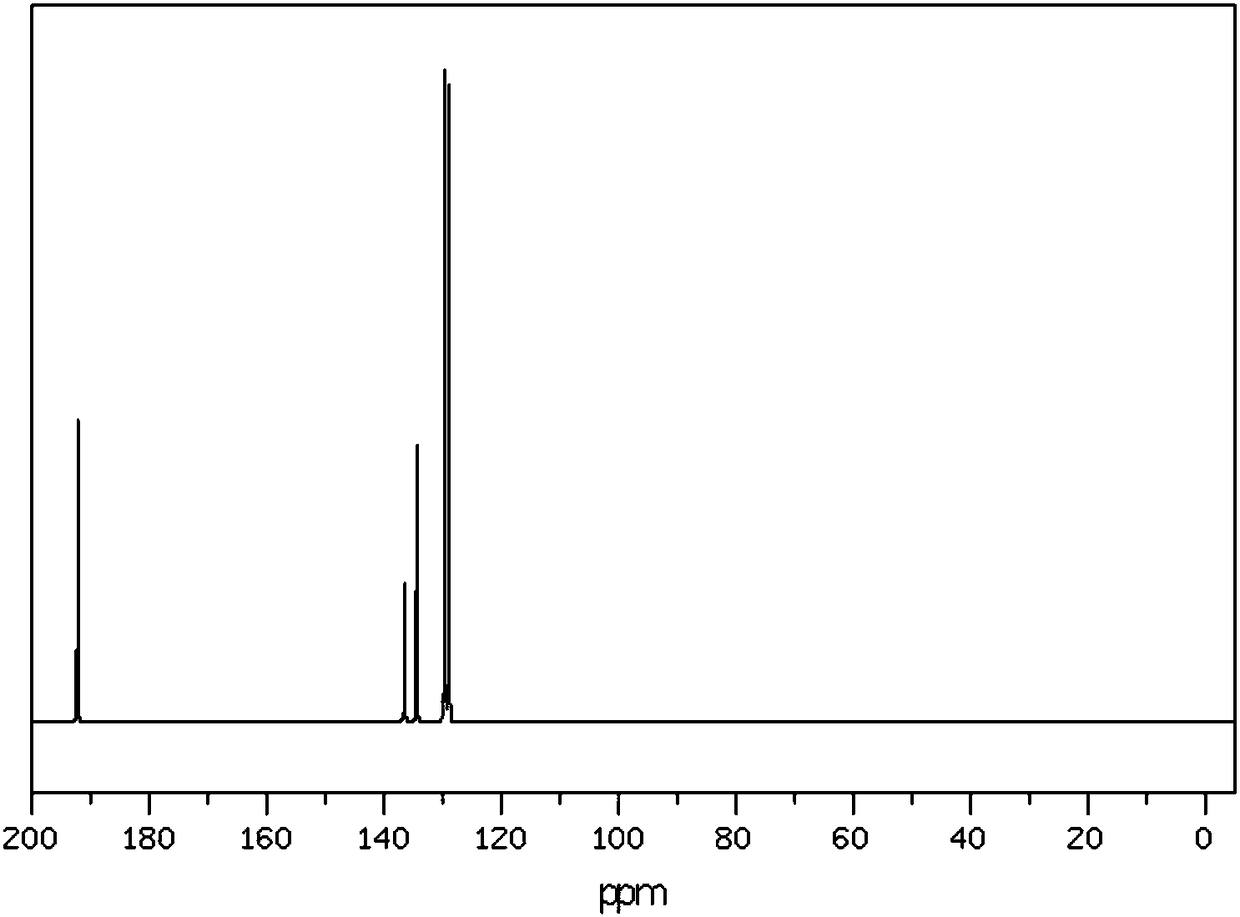
[0029] For finished benzaldehyde 13 C NMR analysis, the analysis spectrum is as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com