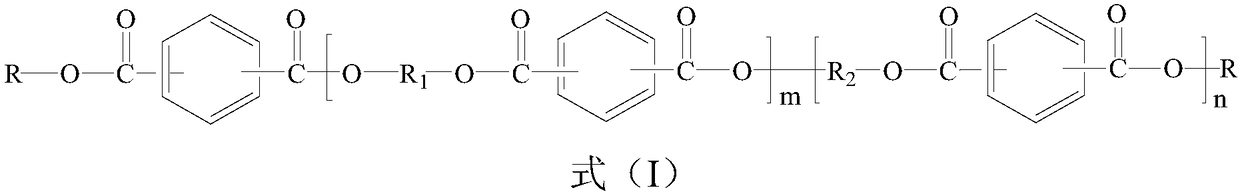
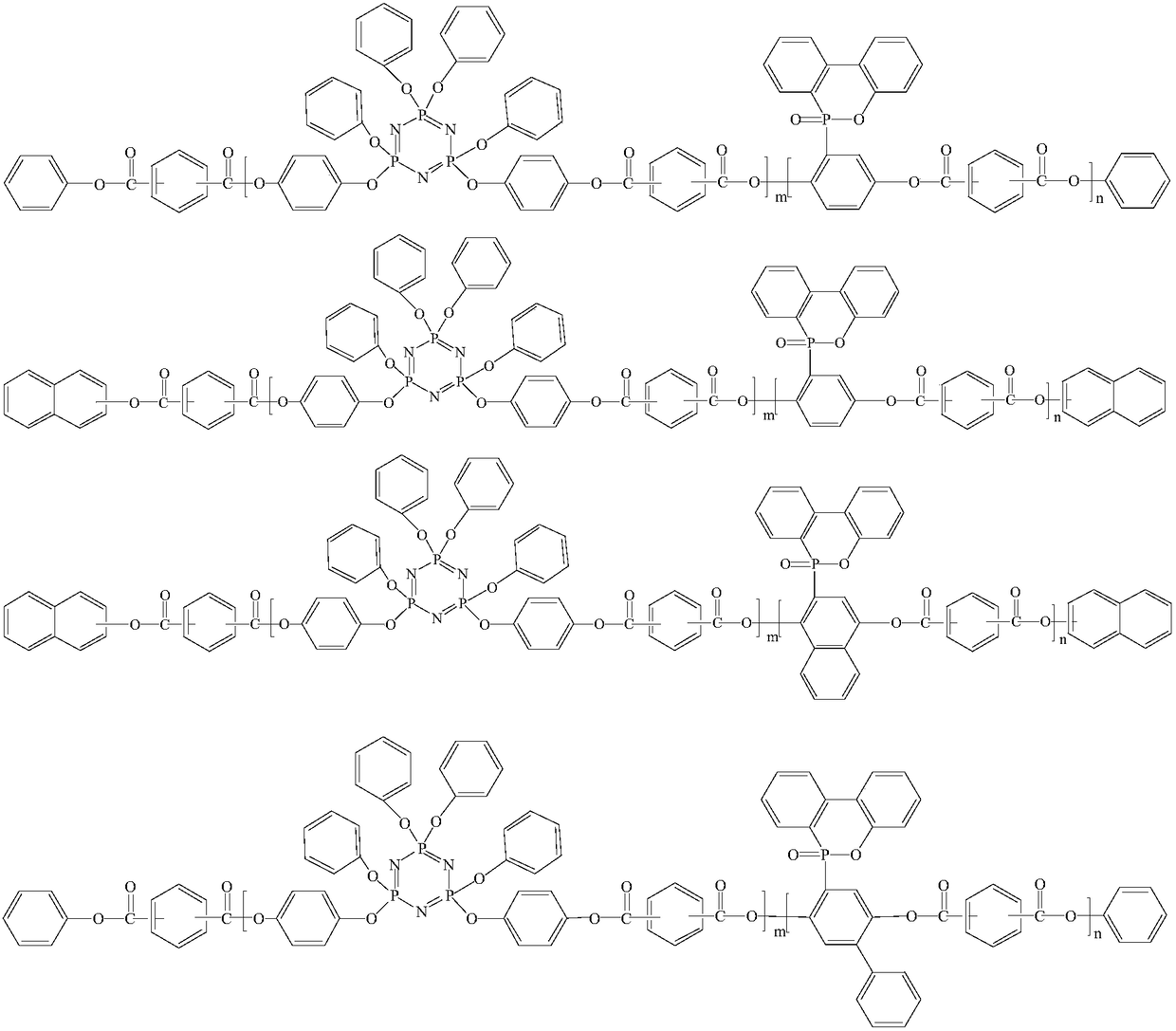
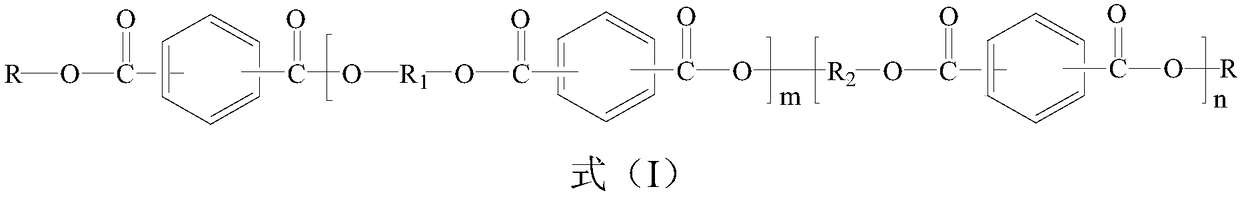
Phosphorus-containing active ester and halogen-free composition thereof, and copper-clad laminate
A technology of active ester and resin composition, applied in the fields of laminates and printed circuit boards, prepregs, phosphorus-containing active esters and their halogen-free resin compositions, can solve the problems of poor dielectric properties, high water absorption, influence Sheet metal performance and other issues, to achieve the effect of meeting halogen-free flame retardant, reducing dielectric loss factor, and improving the electrical properties of the system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0057] Add 160g (0.5mol) 10-(2,5-dihydroxyphenyl)-10-hydrogen-9-oxa-10- Phosphaphenanthrene-10-oxide (ODOPB) and 360g hydroxyl-containing phenoxy cyclotriphosphazene (wherein the dihydroxy content is greater than 60%) and 816g methyl isobutyl ketone (MIBK), nitrogen replacement under reduced pressure in the system , to dissolve it. Then, 182.7g (0.9mol) of terephthaloyl chloride was put into the reaction for 2 hours, and the temperature in the system was controlled below 60°C; then, 114g (1.2mol) of phenol was added to the system, and the reaction was continued for 1 hour; 189 g of 20% aqueous sodium hydroxide solution are added; stirring is continued for 1 hour under these conditions. After the reaction was completed, the water layer was removed by static liquid separation. Add water into the MIBK phase in which the reactants are dissolved, stir and mix, then separate the liquids at rest, and remove the water layer. The above operations were repeated until the pH of the aq...
Synthetic example 2
[0059] Add 185g of 10-(2,5-dihydroxynaphthyl)-10-hydrogen-9-oxa-10-phosphaphenanthrene-10 to a flask equipped with a thermometer, dropping funnel, condenser, fractionating tube, and stirrer -Oxide and 360g of hydroxyl-containing phenoxy cyclotriphosphazene (wherein the dihydroxyl content is greater than 60%) and 816g of methyl isobutyl ketone (MIBK), nitrogen replacement under reduced pressure in the system to make it dissolve. Then, 182.7g (0.9mol) of terephthaloyl chloride was put into the reaction for 2 hours, and the temperature in the system was controlled below 60°C; then, 114g (1.2mol) of phenol was added to the system, and the reaction was continued for 1 hour; 189 g of 20% aqueous sodium hydroxide solution are added; stirring is continued for 1 hour under these conditions. After the reaction was completed, the water layer was removed by static liquid separation. Add water into the MIBK phase in which the reactants are dissolved, stir and mix, then separate the liquid...
Synthetic example 3
[0061] The product of DOPO and phenylbenzoquinone through addition reaction or recrystallized in ethoxyethanol to obtain 10-(2,5-dihydroxybiphenyl)-10-hydrogen-9-oxa-10-phosphorus Heterophenanthrene-10-oxide.
[0062] Add 190g of 10-(2,5-dihydroxybiphenyl)-10-hydrogen-9-oxa-10-phosphaphenanthrene- 10-oxide, 360g of hydroxyl-containing phenoxycyclotriphosphazene (wherein the dihydroxyl content is greater than 60%) and 816g of methyl isobutyl ketone (MIBK), the system is replaced by nitrogen under reduced pressure to dissolve. Then, 182.7g (0.9mol) of terephthaloyl chloride was put into the reaction for 2 hours, and the temperature in the system was controlled below 60°C; then, 114g (1.2mol) of phenol was added to the system, and the reaction was continued for 1 hour; 189 g of 20% aqueous sodium hydroxide solution are added dropwise; stirring is continued under these conditions for 1 hour. After the reaction was completed, the water layer was removed by static liquid separatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com