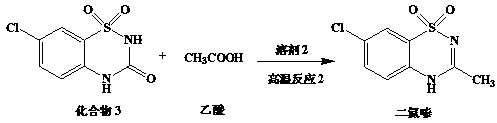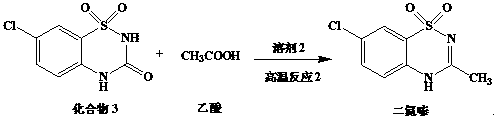Diazoxide preparation method
A technology of diazoxide and compounds, applied in the field of preparation of diazoxide, can solve problems such as unfavorable industrial production, harsh reaction temperature, long reaction route, etc., and achieves less stringent reaction temperature requirements, mild process reaction conditions, and solvent toxicity. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
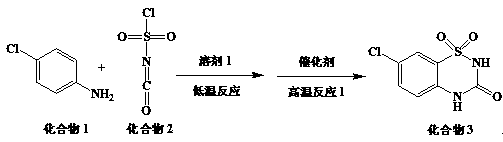
[0032] Preparation of compound 3:
[0033]
[0034] Add p-compound 1 (100g) and chlorobenzene (560ml) into a 1L flask, stir and heat to dissolve, set aside. Add compound 2 (120g) and chlorobenzene (280ml) into a 2L three-necked flask, stir and cool down to 10°C, add the spare p-chloroaniline solution dropwise, and control the temperature during the dropping process at 10°C. Add aluminum trichloride (100g) after dropping, remove the cooling bath, then slowly heat up to 100°C to react for 2 hours, stop heating, and lower the temperature. Prepare another 3L three-necked bottle, add water (1L), and under rapid stirring, slowly pour the reaction solution into water, stir, and suction filter. The filter cake was air-dried for 5 hours to obtain compound 3 with a yield of 59.6% and a purity of 97.7%.
[0035] Preparation of diazoxide:
[0036]
[0037] Add compound 3 (100g), acetic acid (500g) and 98% sulfuric acid (350g) into a 2L three-necked flask and stir, slowly heat to ...
Embodiment 2
[0039] Preparation of compound 3:
[0040]
[0041] Add p-compound 1 (100g) and chlorobenzene (560ml) into a 1L flask, stir and heat to dissolve, set aside. Add compound 2 (120g) and chlorobenzene (280ml) into a 2L three-necked flask, stir and cool down to 10°C, add the spare p-chloroaniline solution dropwise, and control the temperature during the dropping process at -10°C. Add aluminum trichloride (100g) after dropping, remove the cooling bath, then slowly heat up to 100°C to react for 2 hours, stop heating, and lower the temperature. Prepare another 3L three-necked bottle, add water (1L), and under rapid stirring, slowly pour the reaction solution into water, stir, and suction filter. The filter cake was air-dried for 5 hours to obtain compound 3 with a yield of 59.8% and a purity of 98.5%.
[0042] Preparation of diazoxide:
[0043]
[0044] Add compound 3 (100g), acetic acid (500g) and 98% sulfuric acid (350g) into a 2L three-necked flask and stir, slowly heat to 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com