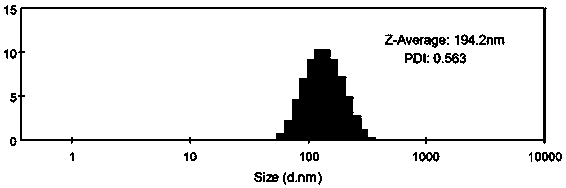
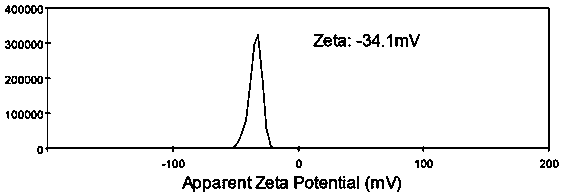
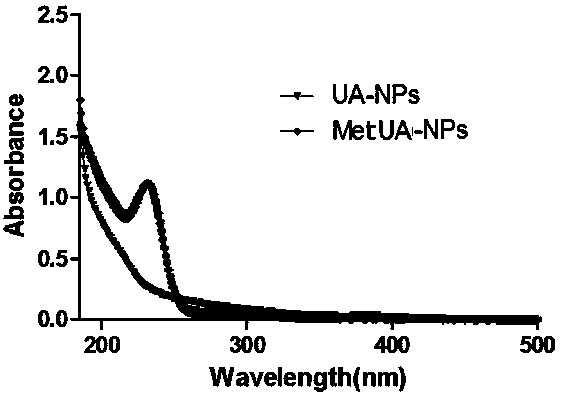
Metformin/ursolic acid nano oral preparation and preparation method thereof
A technology for metformin and oral preparations, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as increased liver burden, increased nephrotoxicity, increased lactic acid, etc. Availability, reduce gastrointestinal irritation, release long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Weigh 33 mg of metformin hydrochloride, dissolve it in 20 mL of distilled water, and make it into an aqueous solution of metformin hydrochloride; weigh 46.5 mg of ursolic acid powder, dissolve it in 5 mL of methanol, and make it into an ursolic acid solution;
[0034] 2) Add 20mL of metformin hydrochloride aqueous solution into a 100mL small beaker, then slowly add 1mL of ursolic acid solution dropwise to it, and stir at room temperature for 1 hour to make it self-assemble to form a metformin / ursolic acid nanoparticle solution;
[0035] 3) Take polyethylene glycol and water to prepare 150ml of 0.1mg / ml polyethylene glycol aqueous solution; mix the obtained metformin / ursolic acid nanoparticle solution with polyethylene glycol aqueous solution and freeze-dry to obtain the drug-containing powder ;
[0036] 4) Add 60 mg of lactose, 15 mg of croscarmellose sodium, 5 mg of silicon dioxide, 5 mg of magnesium stearate and 5 mg of talc into the obtained drug-containing powder...
Embodiment 2
[0038] 1) Weigh 33 mg of metformin hydrochloride, dissolve it in 20 mL of distilled water, and make it into an aqueous solution of metformin hydrochloride; weigh 46.5 mg of ursolic acid powder, dissolve it in 5 mL of methanol, and make it into an ursolic acid solution;
[0039] 2) Add 20mL of metformin hydrochloride aqueous solution into a 100mL small beaker, then slowly add 1mL of ursolic acid solution dropwise to it, and stir at room temperature for 1 hour to make it self-assemble to form a metformin / ursolic acid nanoparticle solution;
[0040] 3) Take polyvinylpyrrolidone and water to form 150ml of 0.1mg / ml polyvinylpyrrolidone aqueous solution; mix the obtained metformin / ursolic acid nanoparticle solution with polyvinylpyrrolidone aqueous solution and freeze-dry to obtain the drug-containing powder;
[0041] 4) Add 60 mg of lactose, 15 mg of crospovidone, 10 mg of silicon dioxide and 5 mg of talc to the obtained drug-containing powder, and mix evenly to obtain the metformin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com